Page 221 - Chemical and process design handbook
P. 221
Speight_Part II_C 11/7/01 3:08 PM Page 2.161
CHLORINE
Chlorine (Cl , melting point: –101°C, boiling point:–34.6°C, density (gas):
2
3.209 g/L at 0°C) is a pale greenish-yellow gas of marked odor, irritating
to the eyes and throat, and poisonous.
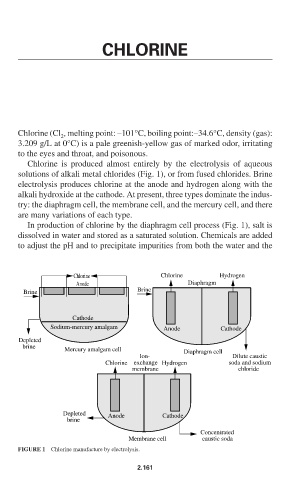
Chlorine is produced almost entirely by the electrolysis of aqueous
solutions of alkali metal chlorides (Fig. 1), or from fused chlorides. Brine
electrolysis produces chlorine at the anode and hydrogen along with the
alkali hydroxide at the cathode. At present, three types dominate the indus-
try: the diaphragm cell, the membrane cell, and the mercury cell, and there
are many variations of each type.
In production of chlorine by the diaphragm cell process (Fig. 1), salt is
dissolved in water and stored as a saturated solution. Chemicals are added
to adjust the pH and to precipitate impurities from both the water and the
Chlorine Chlorine Hydrogen
Anode Diaphragm
Brine Brine
Cathode
Sodium-mercury amalgam Anode Cathode
Depleted
brine
Mercury amalgam cell Diaphragm cell
Ion- Dilute caustic
Chlorine exchange Hydrogen soda and sodium
membrane chloride
Depleted Anode Cathode
brine
Concentrated
Membrane cell caustic soda
FIGURE 1 Chlorine manufacture by electrolysis.
2.161