Page 36 - Chemical and process design handbook
P. 36
Speight_Part 1_H 11/7/01 3:03 PM Page 1.22
1.22 REACTION TYPES
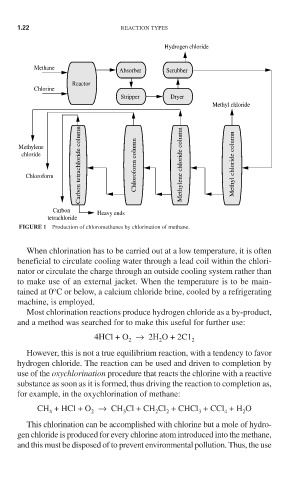
Hydrogen chloride
Methane Absorber Scrubber
Reactor
Chlorine
Stripper Dryer
Methyl chloride
Carbon tetrachloride column Chloroform column Methylene chloride column Methyl chloride column
Methylene
chloride
Chloroform
Carbon Heavy ends
tetrachloride
FIGURE 1 Production of chloromethanes by chlorination of methane.
When chlorination has to be carried out at a low temperature, it is often
beneficial to circulate cooling water through a lead coil within the chlori-
nator or circulate the charge through an outside cooling system rather than
to make use of an external jacket. When the temperature is to be main-
o
tained at 0 C or below, a calcium chloride brine, cooled by a refrigerating
machine, is employed.
Most chlorination reactions produce hydrogen chloride as a by-product,
and a method was searched for to make this useful for further use:
4HCl + O → 2H O + 2C1
2 2 2
However, this is not a true equilibrium reaction, with a tendency to favor
hydrogen chloride. The reaction can be used and driven to completion by
use of the oxychlorination procedure that reacts the chlorine with a reactive
substance as soon as it is formed, thus driving the reaction to completion as,
for example, in the oxychlorination of methane:
CH + HCl + O → CH Cl + CH Cl + CHCl + CCl + H O
4 2 3 2 2 3 4 2
This chlorination can be accomplished with chlorine but a mole of hydro-
gen chloride is produced for every chlorine atom introduced into the methane,
and this must be disposed of to prevent environmental pollution. Thus, the use