Page 63 - Chemical and process design handbook
P. 63
Speight_Part II_A 11/7/01 3:16 PM Page 2.4
2.4 MANUFACTURE OF CHEMICALS
form, but the catalytic species has been shown to be a mercuric ion com-
plex. The excess acetylene sweeps out the dissolved acetaldehyde, which
is condensed by water and refrigerated brine and then scrubbed with water;
this crude acetaldehyde is purified by distillation; the unreacted acetylene
is recycled. The catalytic mercuric ion is reduced to catalytically inactive
mercurous sulfate (Hg SO ) and metallic mercury. Sludge, consisting of
2
4
reduced catalyst and tars, is drained from the reactor at intervals and resul-
fated. The rate of catalyst depletion can be reduced by adding ferric or
other suitable ions to the reaction solution. These ions reoxidize the mer-
curous ion to the mercuric ion; consequently, the quantity of sludge that
must be recovered is reduced.
In one variation of the process, acetylene is completely hydrated with
o
water in a single operation at 68 to 73 C using the mercuric-iron salt cata-
lyst. The acetaldehyde is partially removed by vacuum distillation and the
mother liquor recycled to the reactor. The aldehyde vapors are cooled to
o
about 35 C, compressed to 37 psi (253 kPa), and condensed. It is claimed
that this combination of vacuum and pressure operations substantially
reduces heating and refrigeration costs.
The commercial process of choice for acetaldehyde production is the
direct oxidation of ethylene.
CH =CH + [O] → CH CH=O
2 2 3
There are two variations for this commercial production route: the
two-stage process and the one-stage process.
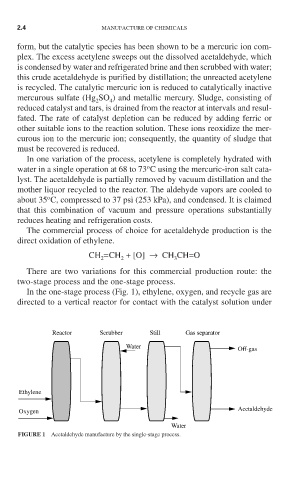
In the one-stage process (Fig. 1), ethylene, oxygen, and recycle gas are
directed to a vertical reactor for contact with the catalyst solution under
Reactor Scrubber Still Gas separator
Water
Off-gas
Ethylene
Oxygen Acetaldehyde
Water
FIGURE 1 Acetaldehyde manufacture by the single-stage process.