Page 64 - Chemical and process design handbook
P. 64
Speight_Part II_A 11/7/01 3:16 PM Page 2.5
ACETALDEHYDE 2.5
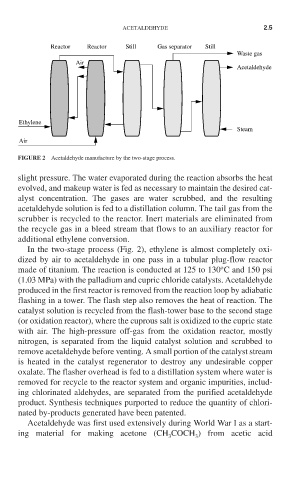
Reactor Reactor Still Gas separator Still
Waste gas
Air
Acetaldehyde
Ethylene
Steam
Air
FIGURE 2 Acetaldehyde manufacture by the two-stage process.
slight pressure. The water evaporated during the reaction absorbs the heat
evolved, and makeup water is fed as necessary to maintain the desired cat-
alyst concentration. The gases are water scrubbed, and the resulting
acetaldehyde solution is fed to a distillation column. The tail gas from the
scrubber is recycled to the reactor. Inert materials are eliminated from
the recycle gas in a bleed stream that flows to an auxiliary reactor for
additional ethylene conversion.
In the two-stage process (Fig. 2), ethylene is almost completely oxi-
dized by air to acetaldehyde in one pass in a tubular plug-flow reactor
o
made of titanium. The reaction is conducted at 125 to 130 C and 150 psi
(1.03 MPa) with the palladium and cupric chloride catalysts. Acetaldehyde
produced in the first reactor is removed from the reaction loop by adiabatic
flashing in a tower. The flash step also removes the heat of reaction. The
catalyst solution is recycled from the flash-tower base to the second stage
(or oxidation reactor), where the cuprous salt is oxidized to the cupric state
with air. The high-pressure off-gas from the oxidation reactor, mostly
nitrogen, is separated from the liquid catalyst solution and scrubbed to
remove acetaldehyde before venting. A small portion of the catalyst stream
is heated in the catalyst regenerator to destroy any undesirable copper
oxalate. The flasher overhead is fed to a distillation system where water is
removed for recycle to the reactor system and organic impurities, includ-
ing chlorinated aldehydes, are separated from the purified acetaldehyde
product. Synthesis techniques purported to reduce the quantity of chlori-
nated by-products generated have been patented.
Acetaldehyde was first used extensively during World War I as a start-
ing material for making acetone (CH COCH ) from acetic acid
3
3