Page 71 - Chemical and process design handbook
P. 71
Speight_Part II_A 11/7/01 3:16 PM Page 2.12
2.12 MANUFACTURE OF CHEMICALS
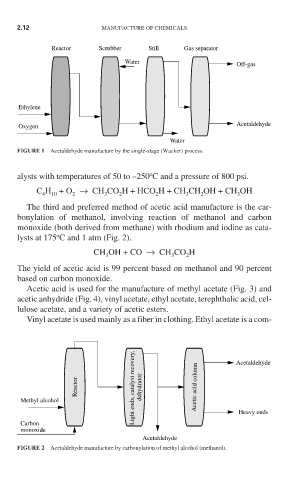
Reactor Scrubber Still Gas separator
Water
Off-gas
Ethylene
Acetaldehyde
Oxygen
Water
FIGURE 1 Acetaldehyde manufacture by the single-stage (Wacker) process.
o
alysts with temperatures of 50 to –250 C and a pressure of 800 psi.
C H + O → CH CO H + HCO H + CH CH OH + CH OH
4 10 2 3 2 2 3 2 3
The third and preferred method of acetic acid manufacture is the car-
bonylation of methanol, involving reaction of methanol and carbon
monoxide (both derived from methane) with rhodium and iodine as cata-
o
lysts at 175 C and 1 atm (Fig. 2).
CH OH + CO → CH CO H
3 3 2
The yield of acetic acid is 99 percent based on methanol and 90 percent
based on carbon monoxide.
Acetic acid is used for the manufacture of methyl acetate (Fig. 3) and
acetic anhydride (Fig. 4), vinyl acetate, ethyl acetate, terephthalic acid, cel-
lulose acetate, and a variety of acetic esters.
Vinyl acetate is used mainly as a fiber in clothing. Ethyl acetate is a com-
Light ends, catalyst recovery, dehydrator Acetic acid column
Reactor Acetaldehyde
Methyl alcohol
Carbon Heavy ends
monoxide
Acetaldehyde
FIGURE 2 Acetaldehyde manufacture by carbonylation of methyl alcohol (methanol).