Page 73 - Chemical and process design handbook
P. 73
Speight_Part II_A 11/7/01 3:16 PM Page 2.14
ACETIC ANHYDRIDE
Acetic anhydride (boiling point: 139.5, density: 1.0820) may be produced
by three different methods. The first procedure involves the in situ pro-
duction from acetaldehyde of peracetic acid, which in turn reacts with
more acetaldehyde to yield the anhydride.
CH CH=O + O → CH C(=O)OH
3
2
3
CH C(=O)OH + CH CH=O → CH C(=O)O(O=)C CH + H O
3
3
3
2
3
In the preferred process, acetic acid (or acetone) is pyrolyzed to ketene,
which reacts with acetic acid to form acetic anhydride.
CH C(=O)OH → CH =C=O + H O
2
2
3
CH =C=O + CH C(=O)OH → CH C(=O)O(O=)C CH 3
3
3
2
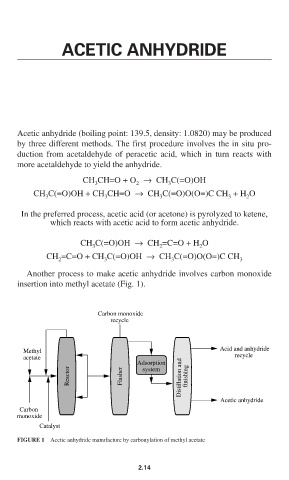
Another process to make acetic anhydride involves carbon monoxide
insertion into methyl acetate (Fig. 1).
Carbon monoxide
recycle
Acid and anhydride
Methyl
acetate recycle
Adsorption
Reactor Flasher Distillation and finishing
system
Acetic anhydride
Carbon
monoxide
Catalyst
FIGURE 1 Acetic anhydride manufacture by carbonylation of methyl acetate
2.14