Page 80 - Chemical and process design handbook
P. 80
Speight_Part II_A 11/7/01 3:16 PM Page 2.21
ACETYLENE 2.21
ing process in which both acetylene and ethylene are end products. Varying
the naphtha feed rate can change the ratio of the two products. Acetylene
also has been produced by a submerged-flame process from crude oil.
o
At 1327 C and higher, acetylene is more stable than other hydrocarbons
but decomposes into its elements. Hence conversion, or splitting, time
must be incredibly short (milliseconds). The amount of energy needed is
very large and in the region of the favorable free energy.
2CH → HC≡CH + 3H
4 2
However, the decomposition of methane (CH ) into its elements starts
4
o
at 578 C, hence competes with its degradation to acetylene.
CH → C + 2H
4 2
To lessen this degradation after raising the methane (or other hydrocar-
o
bon) to a high temperature of about 1500 C for milliseconds, the reaction
mass must be water quenched almost instantaneously.
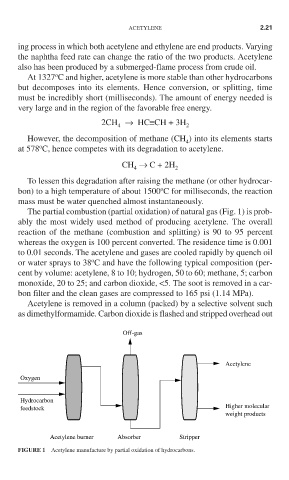
The partial combustion (partial oxidation) of natural gas (Fig. 1) is prob-
ably the most widely used method of producing acetylene. The overall
reaction of the methane (combustion and splitting) is 90 to 95 percent
whereas the oxygen is 100 percent converted. The residence time is 0.001
to 0.01 seconds. The acetylene and gases are cooled rapidly by quench oil
o
or water sprays to 38 C and have the following typical composition (per-
cent by volume: acetylene, 8 to 10; hydrogen, 50 to 60; methane, 5; carbon
monoxide, 20 to 25; and carbon dioxide, <5. The soot is removed in a car-
bon filter and the clean gases are compressed to 165 psi (1.14 MPa).
Acetylene is removed in a column (packed) by a selective solvent such
as dimethylformamide. Carbon dioxide is flashed and stripped overhead out
Off-gas
Acetylene
Oxygen
Hydrocarbon
Higher molecular
feedstock
weight products
Acetylene burner Absorber Stripper
FIGURE 1 Acetylene manufacture by partial oxidation of hydrocarbons.