Page 115 - Chemical engineering design
P. 115
FUNDAMENTALS OF ENERGY BALANCES
If any component undergoes a phase change in the unit, the heat required is computed
Ž
from the latent heat (at 25 C) and the quantity involved. 95
The component specific heat capacity coefficients, A, B, C, D, are stored as a matrix.
If an energy balance is to be made on several units, the specific heat coefficients for all
the components can be entered at the start, and the program rerun for each unit.
The program listing contains sufficient remark statements for the operation of the
program to be easily followed. It is written in GW-BASIC for personal computers. It
can easily be adapted for other forms of BASIC and for use on programmable calcu-
lators. The use of the program is illustrated in Example 3.14a. It has also been used for
other examples in this chapter and in the flow-sheeting, Chapter 4.
Example 3.14a
Use of computer program ENERGY 1
A furnace burns a liquid coal tar fuel derived from coke-ovens. Calculate the heat trans-
ferred in the furnace if the combustion gases leave at 1500 K. The burners operate with
20 per cent excess air.
Ž
Ž
Take the fuel supply temperature as 50 C (323 K) and the air temperature as 15 C
(288 K).
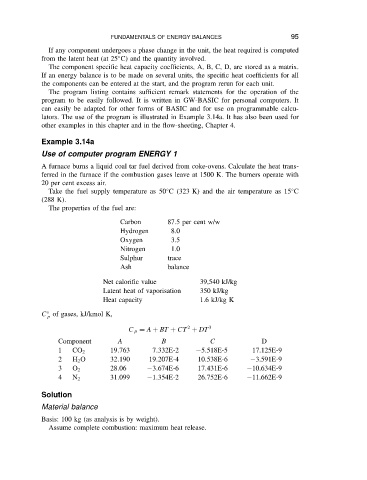
The properties of the fuel are:
Carbon 87.5 per cent w/w
Hydrogen 8.0
Oxygen 3.5
Nitrogen 1.0
Sulphur trace
Ash balance
Net calorific value 39,540 kJ/kg
Latent heat of vaporisation 350 kJ/kg
Heat capacity 1.6 kJ/kg K
Ž
C of gases, kJ/kmol K,
p
2
C p D A C BT C CT C DT 3
Component A B C D
1 CO 2 19.763 7.332E-2 5.518E-5 17.125E-9
2 H 2 O 32.190 19.207E-4 10.538E-6 3.591E-9
3 O 2 28.06 3.674E-6 17.431E-6 10.634E-9
4 N 2 31.099 1.354E-2 26.752E-6 11.662E-9
Solution
Material balance
Basis: 100 kg (as analysis is by weight).
Assume complete combustion: maximum heat release.