Page 370 - Chemical engineering design
P. 370
Solution
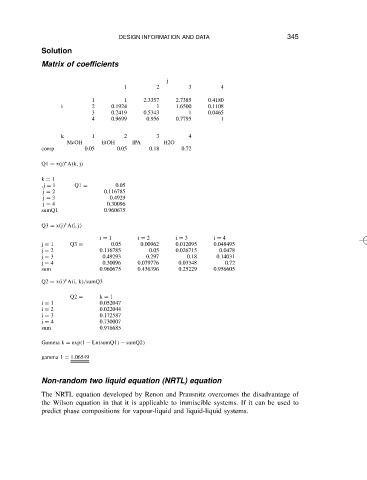
Matrix of coefficients DESIGN INFORMATION AND DATA 345
j
1 2 3 4
1 1 2.3357 2.7385 0.4180
i 2 0.1924 1 1.6500 0.1108
3 0.2419 0.5343 1 0.0465
4 0.9699 0.956 0.7795 1
k 1 2 3 4
MeOH EtOH IPA H2O
comp 0.05 0.05 0.18 0.72
Ł
Q1 D x j A k, j
k D 1
,j D 1 Q1 D 0.05
j D 2 0.116785
j D 3 0.4929
j D 4 0.30096
sumQ1 0.960675
Ł
Q3 D x j A i, j
i D 1 i D 2 i D 3 i D 4
j D 1 Q3 D 0.05 0.00962 0.012095 0.048495
j D 2 0.116785 0.05 0.026715 0.0478
j D 3 0.49293 0.297 0.18 0.14031
j D 4 0.30096 0.079776 0.03348 0.72
sum 0.960675 0.436396 0.25229 0.956605
Ł
Q2 D x i A i, k /sumQ3
Q2 D k D 1
i D 1 0.052047
i D 2 0.022044
i D 3 0.172587
i D 4 0.730007
sum 0.976685
Gamma k D exp 1 Ln sumQ1 sumQ2
gamma 1 D 1.06549
Non-random two liquid equation (NRTL) equation
The NRTL equation developed by Renon and Prausnitz overcomes the disadvantage of
the Wilson equation in that it is applicable to immiscible systems. If it can be used to
predict phase compositions for vapour-liquid and liquid-liquid systems.