Page 158 - Chemical equilibria Volume 4
P. 158
134 Chemical Equilibria
This gives us the value of C P deduced from the measured heat flux.
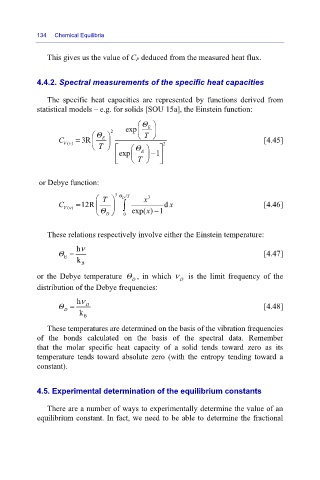
4.4.2. Spectral measurements of the specific heat capacities
The specific heat capacities are represented by functions derived from
statistical models – e.g. for solids [SOU 15a], the Einstein function:
⎛ exp Θ E ⎞
⎛ Θ E ⎞ 2 ⎜ ⎝ T ⎠ ⎟
C Vv = 3R ⎜ ⎟ 2 [4.45]
()
⎝ T ⎠ ⎡ ⎛ Θ E ⎞ ⎤
exp ⎜ ⎢ ⎟ − 1 ⎥
⎝ ⎣ T ⎠ ⎦
or Debye function:
⎛ T ⎞ 3 Θ D /T x 3
C Vv = 12R ⎜ ⎟ ∫ d x [4.46]
x −
()
⎝ Θ D ⎠ 0 exp( ) 1
These relations respectively involve either the Einstein temperature:
hν
Θ = [4.47]
E
k B
or the Debye temperature Θ , in which ν is the limit frequency of the
D
D
distribution of the Debye frequencies:
hν
Θ = D [4.48]
D
k B
These temperatures are determined on the basis of the vibration frequencies
of the bonds calculated on the basis of the spectral data. Remember
that the molar specific heat capacity of a solid tends toward zero as its
temperature tends toward absolute zero (with the entropy tending toward a
constant).
4.5. Experimental determination of the equilibrium constants
There are a number of ways to experimentally determine the value of an
equilibrium constant. In fact, we need to be able to determine the fractional