Page 153 - Chemical equilibria Volume 4
P. 153
Determination of the Values Associated with Reactions – Equilibrium Calculations 129
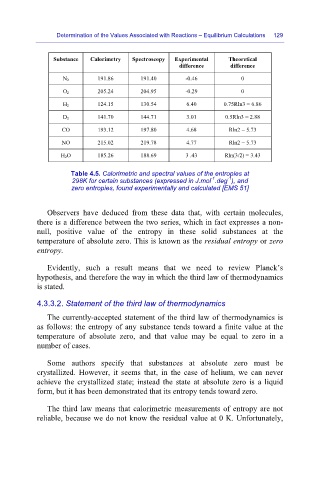
Substance
Calorimetry
Experimental
Spectroscopy
difference Theoretical
difference
N 2 191.86 191.40 -0.46 0
205.24 204.95 -0.29 0
O 2
H 2 124.15 130.54 6.40 0.75Rln3 = 6.86
D 2 141.70 144.71 3.01 0.5Rln3 = 2.88
CO 193.12 197.80 4.68 Rln2 = 5.73
NO 215.02 219.78 4.77 Rln2 = 5.73
H 2O 185.26 188.69 3 .43 Rln(3/2) = 3.43
Table 4.5. Calorimetric and spectral values of the entropies at
-1
-1
298K for certain substances (expressed in J.mol .deg ), and
zero entropies, found experimentally and calculated [EMS 51]
Observers have deduced from these data that, with certain molecules,
there is a difference between the two series, which in fact expresses a non-
null, positive value of the entropy in these solid substances at the
temperature of absolute zero. This is known as the residual entropy or zero
entropy.
Evidently, such a result means that we need to review Planck’s
hypothesis, and therefore the way in which the third law of thermodynamics
is stated.
4.3.3.2. Statement of the third law of thermodynamics
The currently-accepted statement of the third law of thermodynamics is
as follows: the entropy of any substance tends toward a finite value at the
temperature of absolute zero, and that value may be equal to zero in a
number of cases.
Some authors specify that substances at absolute zero must be
crystallized. However, it seems that, in the case of helium, we can never
achieve the crystallized state; instead the state at absolute zero is a liquid
form, but it has been demonstrated that its entropy tends toward zero.
The third law means that calorimetric measurements of entropy are not
reliable, because we do not know the residual value at 0 K. Unfortunately,