Page 151 - Chemical equilibria Volume 4
P. 151
Determination of the Values Associated with Reactions – Equilibrium Calculations 127
This property has been dubbed the third law of thermodynamics.
We can then use relation [4.8] to calculate the standard entropy at any
0
temperature, by making Δ s = and T 0 = 0 K, which gives us:
0
r
0
T Δ C () φ Δ H
T ∑
Δ S = r P dT + ∑ ∫ ν k Δφ (Δφ ) k [4.34]
r
φ (Δφ ) T Δ φ T
T
As the specific heat capacities and the latent heats of state change are
measured by calorimetry, we use the term calorimetric standard entropy to
speak of the value calculated by relation [4.34].
In order to verify the values obtained, we can also determine the values of
the standard entropy when we know the equilibrium constant at the desired
temperature and the standard enthalpy of reaction at the same temperature.
Then by combining expressions [4.1] and [4.2], we obtain:
Δ h 0
Δ s =− Rln K + rT [4.35]
0
rT
P
T
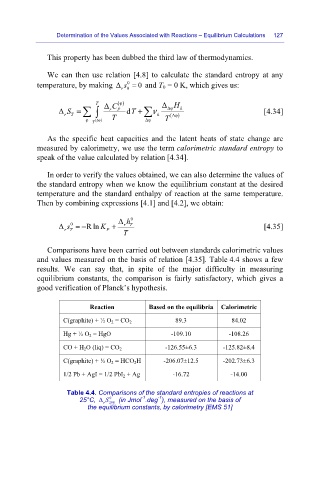
Comparisons have been carried out between standards calorimetric values
and values measured on the basis of relation [4.35]. Table 4.4 shows a few
results. We can say that, in spite of the major difficulty in measuring
equilibrium constants, the comparison is fairly satisfactory, which gives a
good verification of Planck’s hypothesis.
Reaction Based on the equilibria Calorimetric
C(graphite) + ½ O 2 = CO 2 89.3 84.02
Hg + ½ O 2 = HgO -109.10 -108.26
CO + H 2 O (liq) = CO 2 -126.55±6.3 -125.82±8.4
C(graphite) + ½ O 2 = HCO 2 H -206.07±12.5 -202.73±6.3
1/2 Pb + AgI = 1/2 PbI 2 + Ag -16.72 -14.00
Table 4.4. Comparisons of the standard entropies of reactions at
-1
-1
0
25°C, Δ S 298 (in Jmol .deg ), measured on the basis of
r
the equilibrium constants, by calorimetry [EMS 51]