Page 147 - Chemical equilibria Volume 4
P. 147
Determination of the Values Associated with Reactions – Equilibrium Calculations 123
When we deduce the energies of dissociation using the spectral method,
we are able to take account of the fact that the atoms may, following that
dissociation, be in activated states, in which case it is useful to subtract the
activation energy from the value given by the spectrum.
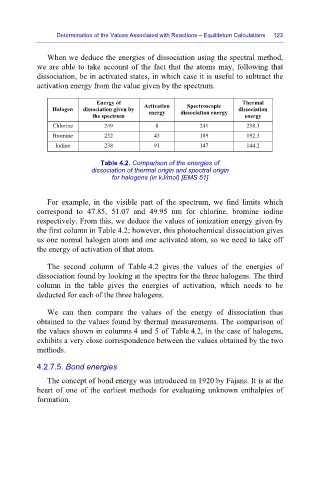
Energy of Activation Spectroscopic Thermal
Halogen dissociation given by dissociation
the spectrum energy dissociation energy energy
Chlorine 249 8 241 238.3
Bromine 232 43 189 192.3
Iodine 238 91 147 144.2
Table 4.2. Comparison of the energies of
dissociation of thermal origin and spectral origin
for halogens (in kJ/mol) [EMS 51]
For example, in the visible part of the spectrum, we find limits which
correspond to 47.85, 51.07 and 49.95 nm for chlorine, bromine iodine
respectively. From this, we deduce the values of ionization energy given by
the first column in Table 4.2; however, this photochemical dissociation gives
us one normal halogen atom and one activated atom, so we need to take off
the energy of activation of that atom.
The second column of Table 4.2 gives the values of the energies of
dissociation found by looking at the spectra for the three halogens. The third
column in the table gives the energies of activation, which needs to be
deducted for each of the three halogens.
We can then compare the values of the energy of dissociation thus
obtained to the values found by thermal measurements. The comparison of
the values shown in columns 4 and 5 of Table 4.2, in the case of halogens,
exhibits a very close correspondence between the values obtained by the two
methods.
4.2.7.5. Bond energies
The concept of bond energy was introduced in 1920 by Fajans. It is at the
heart of one of the earliest methods for evaluating unknown enthalpies of
formation.