Page 142 - Chemical equilibria Volume 4
P. 142
118 Chemical Equilibria
with the sum being extended to the components of the synthesis reaction.
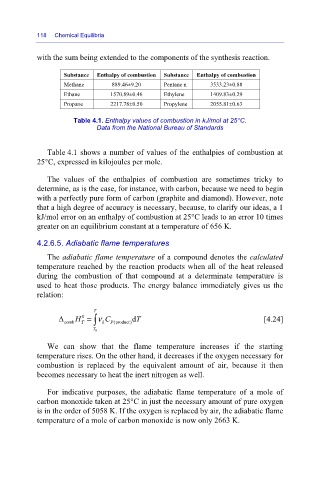
Substance Enthalpy of combustion Substance Enthalpy of combustion
Methane 889.46±9.20 Pentane n 3533.23±0.88
Ethane 1570.89±0.46 Ethylene 1409.83±0.29
Propane 2217.78±0.50 Propylene 2055.81±0.63
Table 4.1. Enthalpy values of combustion in kJ/mol at 25°C.
Data from the National Bureau of Standards
Table 4.1 shows a number of values of the enthalpies of combustion at
25°C, expressed in kilojoules per mole.
The values of the enthalpies of combustion are sometimes tricky to
determine, as is the case, for instance, with carbon, because we need to begin
with a perfectly pure form of carbon (graphite and diamond). However, note
that a high degree of accuracy is necessary, because, to clarify our ideas, a 1
kJ/mol error on an enthalpy of combustion at 25°C leads to an error 10 times
greater on an equilibrium constant at a temperature of 656 K.
4.2.6.5. Adiabatic flame temperatures
The adiabatic flame temperature of a compound denotes the calculated
temperature reached by the reaction products when all of the heat released
during the combustion of that compound at a determinate temperature is
used to heat those products. The energy balance immediately gives us the
relation:
T
T ∫
0
Δ comb H = ν k C P (product) dT [4.24]
0 T
We can show that the flame temperature increases if the starting
temperature rises. On the other hand, it decreases if the oxygen necessary for
combustion is replaced by the equivalent amount of air, because it then
becomes necessary to heat the inert nitrogen as well.
For indicative purposes, the adiabatic flame temperature of a mole of
carbon monoxide taken at 25°C in just the necessary amount of pure oxygen
is in the order of 5058 K. If the oxygen is replaced by air, the adiabatic flame
temperature of a mole of carbon monoxide is now only 2663 K.