Page 170 - Chemical equilibria Volume 4
P. 170
146 Chemical Equilibria
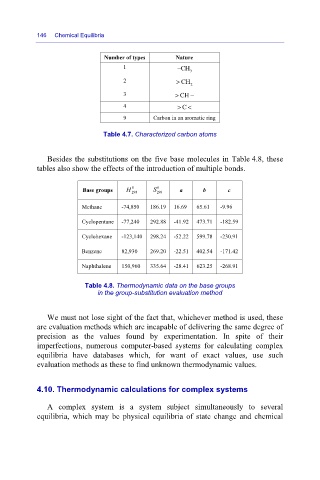
Number of types
−
1 Nature
CH
3
2 > CH
2
3 > CH −
4 > C <
9 Carbon in an aromatic ring
Table 4.7. Characterized carbon atoms
Besides the substitutions on the five base molecules in Table 4.8, these
tables also show the effects of the introduction of multiple bonds.
0
Base groups H 0 298 S 298 a b c
Methane -74,850 186.19 16.69 65.61 -9.96
Cyclopentane -77,240 292.88 -41.92 473.71 -182.59
Cyclohexane -123,140 298.24 -52.22 599.78 -230.91
Benzene 82,930 269.20 -22.51 402.54 -171.42
Naphthalene 150,960 335.64 -28.41 623.25 -268.91
Table 4.8. Thermodynamic data on the base groups
in the group-substitution evaluation method
We must not lose sight of the fact that, whichever method is used, these
are evaluation methods which are incapable of delivering the same degree of
precision as the values found by experimentation. In spite of their
imperfections, numerous computer-based systems for calculating complex
equilibria have databases which, for want of exact values, use such
evaluation methods as these to find unknown thermodynamic values.
4.10. Thermodynamic calculations for complex systems
A complex system is a system subject simultaneously to several
equilibria, which may be physical equilibria of state change and chemical