Page 169 - Chemical equilibria Volume 4
P. 169
Determination of the Values Associated with Reactions – Equilibrium Calculations 145
Berger and Watson. These tables, for a large number of cases, give the result
of a substitution on:
– the standard enthalpy of formation at 298 K;
– the standard entropy at 298 K;
– the coefficients a, b and c of the first three terms in the limited
expansion of the specific heat capacity with temperature.
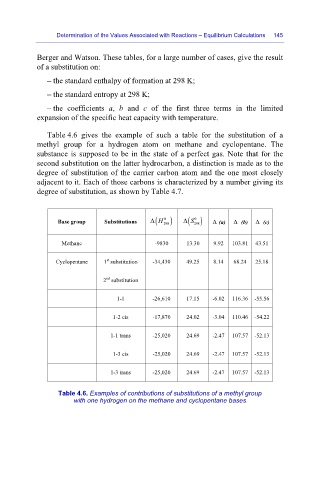
Table 4.6 gives the example of such a table for the substitution of a
methyl group for a hydrogen atom on methane and cyclopentane. The
substance is supposed to be in the state of a perfect gas. Note that for the
second substitution on the latter hydrocarbon, a distinction is made as to the
degree of substitution of the carrier carbon atom and the one most closely
adjacent to it. Each of those carbons is characterized by a number giving its
degree of substitution, as shown by Table 4.7.
Base group Substitutions Δ ( H 0 298 ) Δ S 0 298 Δ (a) Δ (b) Δ (c)
( )
Methane -9830 13.30 9.92 103.81 43.51
st
Cyclopentane 1 substitution -34,430 49.25 8.14 68.24 25.18
nd
2 substitution
1-1 -26,610 17.15 -6.02 116.36 -55.56
1-2 cis -17,870 24.02 -3.04 110.46 -54.22
1-1 trans -25,020 24.69 -2.47 107.57 -52.13
1-3 cis -25,020 24.69 -2.47 107.57 -52.13
1-3 trans -25,020 24.69 -2.47 107.57 -52.13
Table 4.6. Examples of contributions of substitutions of a methyl group
with one hydrogen on the methane and cyclopentane bases