Page 36 - Chemical equilibria Volume 4
P. 36
12 Chemical Equilibria
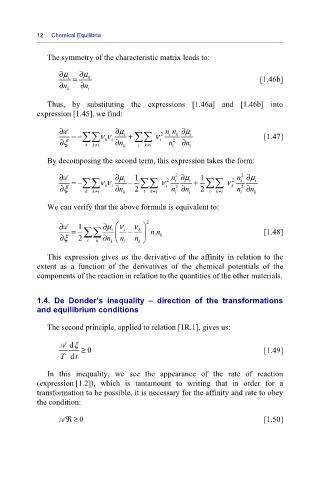
The symmetry of the characteristic matrix leads to:
∂ μ i = ∂ μ k [1.46b]
n ∂ k n ∂ i
Thus, by substituting the expressions [1.46a] and [1.46b] into
expression [1.45], we find:
∂A =− ∑∑ νν ∂μ i + ∑∑ ν 2 nn ∂μ i [1.47]
k
i
∂ξ k k i ≠ ki n ∂ k i k i ≠ i n i 2 n ∂ i
By decomposing the second term, this expression takes the form:
2
2
∂A =− ∑∑ νν ∂μ i − 1 ∑∑ ν 2 n ∂μ i + 1 ∑∑ ν 2 n ∂μ i
i
i
2
∂ξ k k i ≠ ki n ∂ k 2 i k i ≠ i n ∂ n i 2 i k i ≠ k n ∂ n k
2
i
i
We can verify that the above formula is equivalent to:
⎛
∂A = 1 ∂μν i − ν k ⎞ 2 nn [1.48]
⎜ ∑∑
i
∂ξ 2 i k ∂ n k ⎝ n i n k ⎠ ⎟ ik
This expression gives us the derivative of the affinity in relation to the
extent as a function of the derivatives of the chemical potentials of the
components of the reaction in relation to the quantities of the other materials.
1.4. De Donder’s inequality – direction of the transformations
and equilibrium conditions
The second principle, applied to relation [1R.1], gives us:
A dξ ≥
T dt 0 [1.49]
In this inequality, we see the appearance of the rate of reaction
(expression [1.2]), which is tantamount to writing that in order for a
transformation to be possible, it is necessary for the affinity and rate to obey
the condition:
A ℜ≥ 0 [1.50]