Page 112 - Chemical process engineering design and economics
P. 112
96 Chapter 3
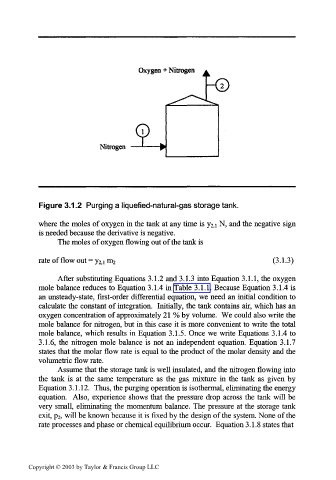
Oxygen + Nitrogen
Nitrogen
Figure 3.1.2 Purging a liquefied-natural-gas storage tank.
where the moles of oxygen in the tank at any time is y,i N, and the negative sign
2
is needed because the derivative is negative.
The moles of oxygen flowing out of the tank is
rate of flow out = y>,i (3.1.3)
After substituting Equations 3.1.2 and 3.1.3 into Equation 3.1.1, the oxygen
mole balance reduces to Equation 3.1.4 in Table 3.1.1. Because Equation 3.1.4 is
an unsteady-state, first-order differential equation, we need an initial condition to
calculate the constant of integration. Initially, the tank contains air, which has an
oxygen concentration of approximately 21 % by volume. We could also write the
mole balance for nitrogen, but in this case it is more convenient to write the total
mole balance, which results in Equation 3.1.5. Once we write Equations 3.1.4 to
3.1.6, the nitrogen mole balance is not an independent equation. Equation 3.1.7
states that the molar flow rate is equal to the product of the molar density and the
volumetric flow rate.
Assume that the storage tank is well insulated, and the nitrogen flowing into
the tank is at the same temperature as the gas mixture in the tank as given by
Equation 3.1.12. Thus, the purging operation is isothermal, eliminating the energy
equation. Also, experience shows that the pressure drop across the tank will be
very small, eliminating the momentum balance. The pressure at the storage tank
exit, p 2, will be known because it is fixed by the design of the system. None of the
rate processes and phase or chemical equilibrium occur. Equation 3.1.8 states that
Copyright © 2003 by Taylor & Francis Group LLC