Page 138 - Chemical process engineering design and economics
P. 138
122 Chapter 3
After substituting Equation 3.4.3 into Equation 3.4.2, we obtain Equation
3.4.12 in Table 3.4.1. Physically, Equation 3.4.12 means that the enthalpy flowing
into the reactor with the reactant stream plus part of the enthalpy released in the
reactor by chemical reaction will raise the temperature of the products to 600 °C.
The coolant removes the remaining enthalpy of reaction as heat. For simplicity, we
again assume that we can use the mole fraction average of the pure component
enthalpies for the enthapy of gas mixtures as given by Equations 3.4.13 and
3.4.14. Equations 3.4.15 to 3.4.22 are the pure component enthalpies we need for
Equations 3.4.13 and 3.4.14.
The reactor analysis given in Table 3.4.1 shows that there are two degrees of
freedom, and thus we have not completely defined the problem. We must either
write two additional equations or specify two additional variables. In this case, we
see that in Figure 3.4.1 the methanol and air streams mix before entering the reac-
tor. Mixing is a process step even though the mixer may only be two intersecting
streams. Table 3.4.2 lists the equations for the mixer, which are three additional
mole balances. The equations, however, contain an additional variable, m 2. We
have already written the mole fraction summation for stream 3. The air and
methanol streams are at the same temperature so that we do not need an energy
balance for the mixer.
Ah
Ah*
Ah 3
Ah R
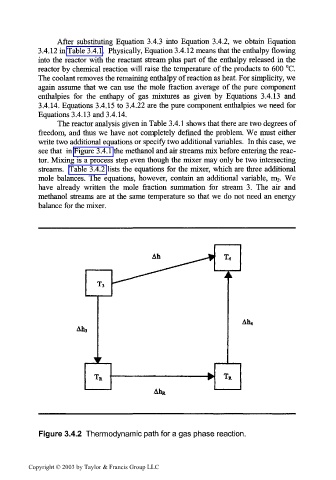
Figure 3.4.2 Thermodynamic path for a gas phase reaction.
Copyright © 2003 by Taylor & Francis Group LLC