Page 142 - Chemical process engineering design and economics
P. 142
126 Chapter 3
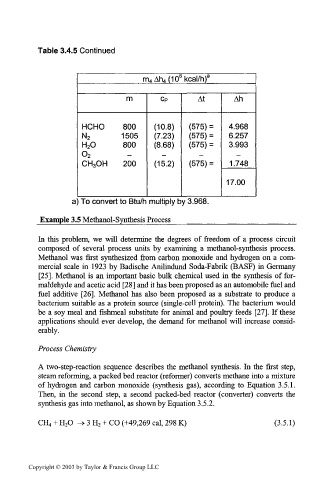
Table 3.4.5 Continued
6
m 4Ah 4(10 kcal/h) a
m CP At Ah
HCHO 800 (10.8) (575) = 4.968
(7.23)
N 2 1505 (575) = 6.257
H 2O 800 (8.68) (575) = 3.993
0 2
CH 3OH 200 (15.2) (575) = 1.748
17.00
a) To convert to Btu/h multiply by 3.968.
Example 3.5 Methanol-Synthesis Process______________________
In this problem, we will determine the degrees of freedom of a process circuit
composed of several process units by examining a methanol-synthesis process.
Methanol was first synthesized from carbon monoxide and hydrogen on a com-
mercial scale in 1923 by Badische Anilindund Soda-Fabrik (BASF) in Germany
[25]. Methanol is an important basic bulk chemical used in the synthesis of for-
maldehyde and acetic acid [28] and it has been proposed as an automobile fuel and
fuel additive [26]. Methanol has also been proposed as a substrate to produce a
bacterium suitable as a protein source (single-cell protein). The bacterium would
be a soy meal and fishmeal substitute for animal and poultry feeds [27]. If these
applications should ever develop, the demand for methanol will increase consid-
erably.
Process Chemistry
A two-step-reaction sequence describes the methanol synthesis. In the first step,
steam reforming, a packed bed reactor (reformer) converts methane into a mixture
of hydrogen and carbon monoxide (synthesis gas), according to Equation 3.5.1.
Then, in the second step, a second packed-bed reactor (converter) converts the
synthesis gas into methanol, as shown by Equation 3.5.2.
CH 4 + H 2O -> 3 H 2 + CO (+49,269 cal, 298 K) (3.5.1)
Copyright © 2003 by Taylor & Francis Group LLC