Page 311 - Chiral Separation Techniques
P. 311
12.3 Advantages of SFC for Chiral Separations 303
higher in SFC, and analysis times were shorter compared to LC. They also explored
the influence of the type of modifier on selectivity in SFC.
Mourier’s report was quickly followed by successful enantiomeric resolutions on
stationary phases bearing other types of chiral selectors, including native and deriva-
tized cyclodextrins and derivatized polysaccharides. Many chiral compounds of
pharmaceutical interest have now been resolved by packed column SFC, including
antimalarials, β-blockers, and antivirals. A summary is provided in Table 12-2. Most
of the applications have utilized modified CO as the eluent.
2
Table 12-2. Selected applications of chiral SFC to pharmaceutical compounds . a
Chiral stationary phase Compounds resolved
Cellulose derivatives β-Blockers, benzodiazepines, NSAIDs, barbiturates
Amylose derivatives NSAIDs, protease inhibitors, β-blockers, benzodiazepines
Brush-type Antimalarials, NSAIDs, β-blockers, bronchodilators
Cyclodextrins and derivatives Phosphine oxides, NSAIDs, anticonvulsants
Macrocyclic antibiotics Bronchodilators, β-blockers
a For a more comprehensive listing, see. ref. [12].
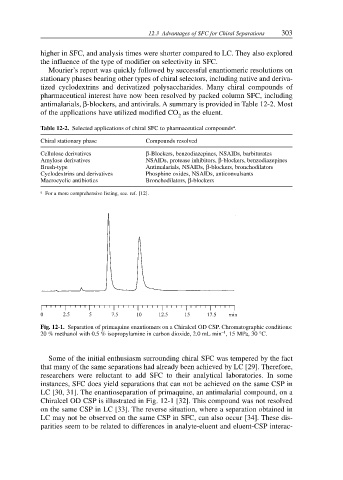
Fig. 12-1. Separation of primaquine enantiomers on a Chiralcel OD CSP. Chromatographic conditions:
–1
20 % methanol with 0.5 % isopropylamine in carbon dioxide, 2.0 mL min , 15 MPa, 30 °C.
Some of the initial enthusiasm surrounding chiral SFC was tempered by the fact
that many of the same separations had already been achieved by LC [29]. Therefore,
researchers were reluctant to add SFC to their analytical laboratories. In some
instances, SFC does yield separations that can not be achieved on the same CSP in
LC [30, 31]. The enantioseparation of primaquine, an antimalarial compound, on a
Chiralcel OD CSP is illustrated in Fig. 12-1 [32]. This compound was not resolved
on the same CSP in LC [33]. The reverse situation, where a separation obtained in
LC may not be observed on the same CSP in SFC, can also occur [34]. These dis-
parities seem to be related to differences in analyte-eluent and eluent-CSP interac-