Page 316 - Chiral Separation Techniques
P. 316
308 12 Sub- and Supercritical Fluid Chromatography for Enantiomer Separations
12.4.2 Cyclodextrins
Cyclodextrins are cyclic oligosaccharides comprised of glucose units joined through
α-1,4 linkages. In LC, chiral recognition is believed to involve the formation of
inclusion complexes between the analyte and the hydrophobic cavity of the
cyclodextrin. The use of aqueous-organic mobile phases facilitates complex forma-
tion. Under normal phase conditions, the apolar component of the mobile phase
occupies the cyclodextrin cavity, reducing opportunities for chiral complexation
[51]. Cyclodextrin-based CSPs have also been used in the polar organic mode. In
this mode, the analyte is believed to interact primarily with the hydroxyl groups
along the mouth of the cyclodextrin [52].
Macaudière et al. first reported the enantiomeric separation of racemic phosphine
oxides and amides on native cyclodextrin-based CSPs under subcritical conditions
[53]. The separations obtained were indicative of inclusion complexation. When the
CO –methanol eluent used in SFC was replaced with hexane-ethanol in LC, reduced
2
selectivity was observed. The authors proposed that the smaller size of the CO
2
molecule made it less likely than hexane to compete with the analyte for the
cyclodextrin cavity.
Comparisons of LC and SFC have also been performed on naphthylethylcar-
bamoylated-β-cyclodextrin CSPs. These multimodal CSPs can be used in conjunc-
tion with normal phase, reversed phase, and polar organic eluents. Discrete sets of
chiral compounds tend to be resolved in each of the three mobile phase modes in LC.
As demonstrated by Williams et al., separations obtained in each of the different
mobile phase modes in LC could be replicated with a simple CO -methanol eluent
2
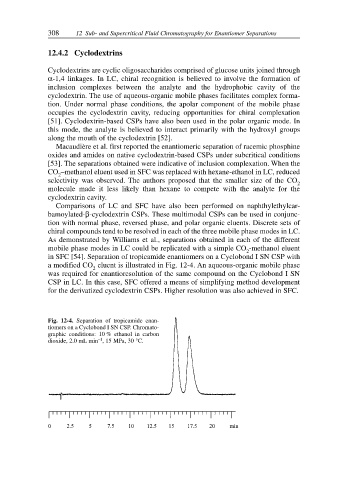
in SFC [54]. Separation of tropicamide enantiomers on a Cyclobond I SN CSP with
a modified CO eluent is illustrated in Fig. 12-4. An aqueous-organic mobile phase
2
was required for enantioresolution of the same compound on the Cyclobond I SN
CSP in LC. In this case, SFC offered a means of simplifying method development
for the derivatized cyclodextrin CSPs. Higher resolution was also achieved in SFC.
Fig. 12-4. Separation of tropicamide enan-
tiomers on a Cyclobond I SN CSP. Chromato-
graphic conditions: 10 % ethanol in carbon
–1
dioxide, 2.0 mL min , 15 MPa, 30 °C.