Page 325 - Chiral Separation Techniques
P. 325
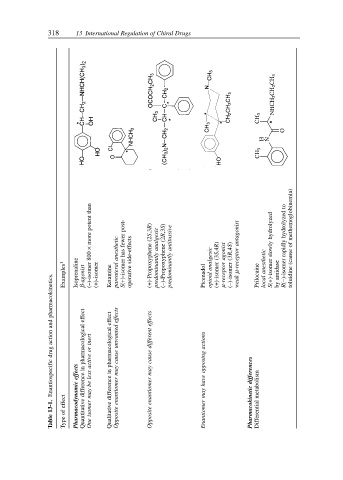
318 13 International Regulation of Chiral Drugs
more potent than β-agonist (–)-isomer 800 × (+)-isomer Ketamine parenteral anesthetic S(+)-isomer has fewer post- operative side-effects (+)-Propoxyphene (2S,3R) predominantly analgesic (–)-Propoxyphene (2R,3S) predominantly antitussive Picenadol opioid analgesic (+)-isomer (3S,4R) µ-receptor agonist (–)-isomer (3R,4S) µ-receptor antagonist Prilo
Isoprenaline
Examples 1
Enantiospecific drug action and pharmacokinetics. Quantitative difference in pharmacological effect Qualitative difference in pharmacological effect Opposite enantiomer may cause unwanted effects Opposite enantiomer may cause different effects weak
Table 13-1. Type of effect Pharmacodynamic effects One isomer may be less active or inert Enantiomer may have opposing actions Pharmacokinetic differences Differential metabolism