Page 341 - Chiral Separation Techniques
P. 341
334 13 International Regulation of Chiral Drugs
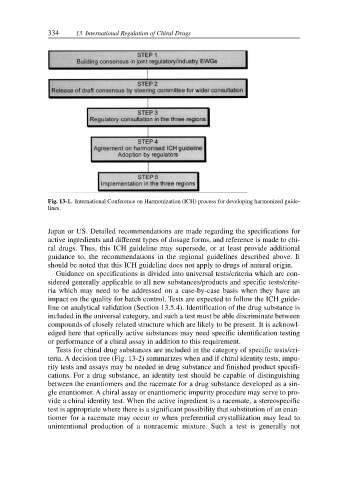
Fig. 13-1. International Conference on Harmonization (ICH) process for developing harmonized guide-
lines.
Japan or US. Detailed recommendations are made regarding the specifications for
active ingredients and different types of dosage forms, and reference is made to chi-
ral drugs. Thus, this ICH guideline may supersede, or at least provide additional
guidance to, the recommendations in the regional guidelines described above. It
should be noted that this ICH guideline does not apply to drugs of natural origin.
Guidance on specifications is divided into universal tests/criteria which are con-
sidered generally applicable to all new substances/products and specific tests/crite-
ria which may need to be addressed on a case-by-case basis when they have an
impact on the quality for batch control. Tests are expected to follow the ICH guide-
line on analytical validation (Section 13.5.4). Identification of the drug substance is
included in the universal category, and such a test must be able discriminate between
compounds of closely related structure which are likely to be present. It is acknowl-
edged here that optically active substances may need specific identification testing
or performance of a chiral assay in addition to this requirement.
Tests for chiral drug substances are included in the category of specific tests/cri-
teria. A decision tree (Fig. 13-2) summarizes when and if chiral identity tests, impu-
rity tests and assays may be needed in drug substance and finished product specifi-
cations. For a drug substance, an identity test should be capable of distinguishing
between the enantiomers and the racemate for a drug substance developed as a sin-
gle enantiomer. A chiral assay or enantiomeric impurity procedure may serve to pro-
vide a chiral identity test. When the active ingredient is a racemate, a stereospecific
test is appropriate where there is a significant possibility that substitution of an enan-
tiomer for a racemate may occur or when preferential crystallization may lead to
unintentional production of a nonracemic mixture. Such a test is generally not