Page 342 - Chiral Separation Techniques
P. 342
13.5 Guidelines from the International Conference on Harmonization 335
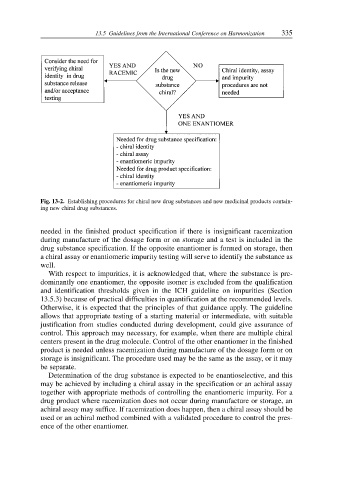
Fig. 13-2. Establishing procedures for chiral new drug substances and new medicinal products contain-
ing new chiral drug substances.
needed in the finished product specification if there is insignificant racemization
during manufacture of the dosage form or on storage and a test is included in the
drug substance specification. If the opposite enantiomer is formed on storage, then
a chiral assay or enantiomeric impurity testing will serve to identify the substance as
well.
With respect to impurities, it is acknowledged that, where the substance is pre-
dominantly one enantiomer, the opposite isomer is excluded from the qualification
and identification thresholds given in the ICH guideline on impurities (Section
13.5.3) because of practical difficulties in quantification at the recommended levels.
Otherwise, it is expected that the principles of that guidance apply. The guideline
allows that appropriate testing of a starting material or intermediate, with suitable
justification from studies conducted during development, could give assurance of
control. This approach may necessary, for example, when there are multiple chiral
centers present in the drug molecule. Control of the other enantiomer in the finished
product is needed unless racemization during manufacture of the dosage form or on
storage is insignificant. The procedure used may be the same as the assay, or it may
be separate.
Determination of the drug substance is expected to be enantioselective, and this
may be achieved by including a chiral assay in the specification or an achiral assay
together with appropriate methods of controlling the enantiomeric impurity. For a
drug product where racemization does not occur during manufacture or storage, an
achiral assay may suffice. If racemization does happen, then a chiral assay should be
used or an achiral method combined with a validated procedure to control the pres-
ence of the other enantiomer.