Page 42 - Chiral Separation Techniques
P. 42
1.5 Other Methods 17
chromatographic supports impregnated with chiral selectors [195–197]. However,
these techniques seem, at present, far from being applicable to the resolution of
important amounts of racemates.
1.5.3 Enantioselective Distillation and Foam Flotation
Among the existing separation techniques, some – due to their intrinsic characteris-
tics – are more adapted than others to processing large amounts of material. Such pro-
cesses, which already exist at industrial level, can be considered in order to perform
an enantioselective separation. This is the case for techniques such as distillation and
foam flotation, both of which constitute well-known techniques that can be adapted
to the separation of enantiomers. The involvement of a chiral selector can be the clue
which changes a nonstereoselective process into an enantioselective one. Clearly, this
selector must be adapted to the characteristics and limitations of the process itself.
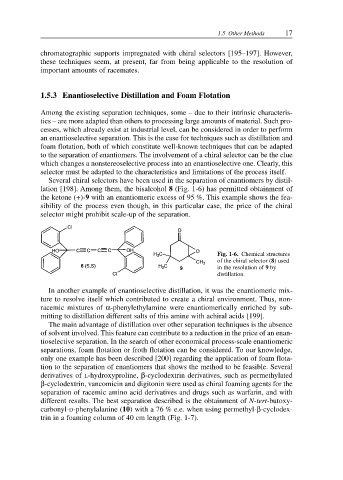
Several chiral selectors have been used in the separation of enantiomers by distil-
lation [198]. Among them, the bisalcohol 8 (Fig. 1-6) has permitted obtainment of
the ketone (+)-9 with an enantiomeric excess of 95 %. This example shows the fea-
sibility of the process even though, in this particular case, the price of the chiral
selector might prohibit scale-up of the separation.
Fig. 1-6. Chemical structures
of the chiral selector (8) used
in the resolution of 9 by
distillation.
In another example of enantioselective distillation, it was the enantiomeric mix-
ture to resolve itself which contributed to create a chiral environment. Thus, non-
racemic mixtures of α-phenylethylamine were enantiomerically enriched by sub-
mitting to distillation different salts of this amine with achiral acids [199].
The main advantage of distillation over other separation techniques is the absence
of solvent involved. This feature can contribute to a reduction in the price of an enan-
tioselective separation. In the search of other economical process-scale enantiomeric
separations, foam flotation or froth flotation can be considered. To our knowledge,
only one example has been described [200] regarding the application of foam flota-
tion to the separation of enantiomers that shows the method to be feasible. Several
derivatives of L-hydroxyproline, β-cyclodextrin derivatives, such as permethylated
β-cyclodextrin, vancomicin and digitonin were used as chiral foaming agents for the
separation of racemic amino acid derivatives and drugs such as warfarin, and with
different results. The best separation described is the obtainment of N-tert-butoxy-
carbonyl-D-phenylalanine (10) with a 76 % e.e. when using permethyl-β-cyclodex-
trin in a foaming column of 40 cm length (Fig. 1-7).