Page 43 - Chiral Separation Techniques
P. 43
18 1 Techniques in preparative chiral separations
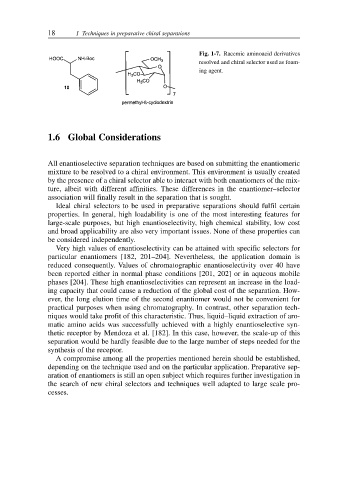
Fig. 1-7. Racemic aminoacid derivatives
resolved and chiral selector used as foam-
ing agent.
1.6 Global Considerations
All enantioselective separation techniques are based on submitting the enantiomeric
mixture to be resolved to a chiral environment. This environment is usually created
by the presence of a chiral selector able to interact with both enantiomers of the mix-
ture, albeit with different affinities. These differences in the enantiomer–selector
association will finally result in the separation that is sought.
Ideal chiral selectors to be used in preparative separations should fulfil certain
properties. In general, high loadability is one of the most interesting features for
large-scale purposes, but high enantioselectivity, high chemical stability, low cost
and broad applicability are also very important issues. None of these properties can
be considered independently.
Very high values of enantioselectivity can be attained with specific selectors for
particular enantiomers [182, 201–204]. Nevertheless, the application domain is
reduced consequently. Values of chromatographic enantioselectivity over 40 have
been reported either in normal phase conditions [201, 202] or in aqueous mobile
phases [204]. These high enantioselectivities can represent an increase in the load-
ing capacity that could cause a reduction of the global cost of the separation. How-
ever, the long elution time of the second enantiomer would not be convenient for
practical purposes when using chromatography. In contrast, other separation tech-
niques would take profit of this characteristic. Thus, liquid–liquid extraction of aro-
matic amino acids was successfully achieved with a highly enantioselective syn-
thetic receptor by Mendoza et al. [182]. In this case, however, the scale-up of this
separation would be hardly feasible due to the large number of steps needed for the
synthesis of the receptor.
A compromise among all the properties mentioned herein should be established,
depending on the technique used and on the particular application. Preparative sep-
aration of enantiomers is still an open subject which requires further investigation in
the search of new chiral selectors and techniques well adapted to large scale pro-
cesses.