Page 102 - Color Atlas of Biochemistry
P. 102
Enzymes 93
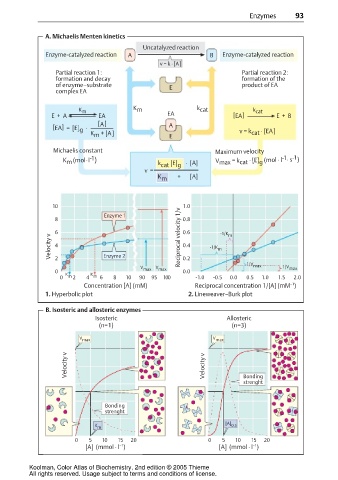
A. Michaelis Menten kinetics
Uncatalyzed reaction
Enzyme-catalyzed reaction A B Enzyme-catalyzed reaction
v = k · [A]
Partial reaction 1: Partial reaction 2:
formation and decay formation of the
of enzyme–substrate E product of EA
complex EA
K m K m k cat k cat
E + A EA EA [EA] E + B
[A ] A
[EA] = [E] ·
g v = k · [EA]
K + [A] E cat
m
Michaelis constant Maximum velocity
-1 -1
-1
K m (mol · l ) k cat [E] g · [A] V max = k cat · [E] (mol · l · s )
g
v =
K m + [A]
10 1.0
Enzyme 1
8 0.8
6 Reciprocal velocity 1/v 0.6 -1/K m
Velocity v 4 Enzyme 2 0.4 -1/K m
2
V V 0.2 1/V max 1/V max
0 K K max max 0.0
0 m 2 4 m 6 8 10 90 95 100 -1.0 -0.5 0.0 0.5 1.0 1.5 2.0
–1
Concentration [A] (mM) Reciprocal concentration 1/[A] (mM )
1. Hyperbolic plot 2. Lineweaver–Burk plot
B. Isosteric and allosteric enzymes
Isosteric Allosteric
(n=1) (n=3)
V max V max
Velocity v Velocity v
Bonding
strenght
Bonding
strenght
K [A] O.5
m
0 5 10 15 20 0 5 10 15 20
–1
–1
[A] (mmol · l ) [A] (mmol · l )
Koolman, Color Atlas of Biochemistry, 2nd edition © 2005 Thieme
All rights reserved. Usage subject to terms and conditions of license.