Page 369 - Corrosion Engineering Principles and Practice
P. 369
338 C h a p t e r 9 A t m o s p h e r i c C o r r o s i o n 339
9.3.1 Relative Humidity and Dew Point
Relative humidity is defined as the ratio of the quantity of water vapor
present in the atmosphere to the saturation quantity at a given temperature,
and it is expressed as percent. A fundamental requirement for atmospheric
corrosion processes is the presence of a thin film electrolyte that can form
on metallic surfaces when exposed to a critical level of humidity. While
this film is almost invisible, the corrosive contaminants it contains are
known to reach relatively high concentrations, especially under conditions
of alternate wetting and drying.
The critical humidity level itself is a variable that depends on the
nature of the corroding material, the tendency of corrosion products and
surface deposits to absorb moisture, and the presence of atmospheric
pollutants [3]. It has been shown, for example, that this critical humidity
level is 60 percent for steel when the environment is free of pollutants.
In the presence of thin film electrolytes, atmospheric corrosion
proceeds by balanced anodic and cathodic reactions described,
respectively, in Eqs. (9.1) and (9.2). The anodic oxidation reaction
involves the corrosion attack of the metal, while the cathodic reaction
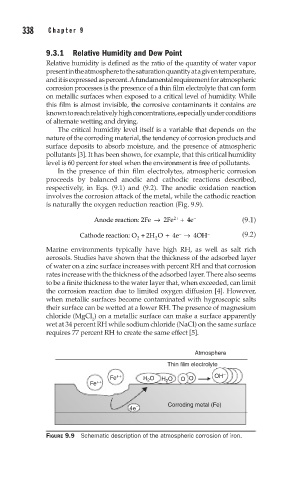
is naturally the oxygen reduction reaction (Fig. 9.9).
−
Anode reaction: 2Fe → 2Fe 2+ + 4e (9.1)
−
Cathode reaction: O + 2H O + 4e → 4OH (9.2)
−
2 2
Marine environments typically have high RH, as well as salt rich
aerosols. Studies have shown that the thickness of the adsorbed layer
of water on a zinc surface increases with percent RH and that corrosion
rates increase with the thickness of the adsorbed layer. There also seems
to be a finite thickness to the water layer that, when exceeded, can limit
the corrosion reaction due to limited oxygen diffusion [4]. However,
when metallic surfaces become contaminated with hygroscopic salts
their surface can be wetted at a lower RH. The presence of magnesium
chloride (MgCl ) on a metallic surface can make a surface apparently
2
wet at 34 percent RH while sodium chloride (NaCl) on the same surface
requires 77 percent RH to create the same effect [5].
Atmosphere
Thin film electrolyte
Fe ++ H O H 2 O O O OH –
2
Fe ++
4e – Corroding metal (Fe)
FIGURE 9.9 Schematic description of the atmospheric corrosion of iron.