Page 668 - Corrosion Engineering Principles and Practice
P. 668
622 C h a p t e r 1 4 P r o t e c t i v e C o a t i n g s 623
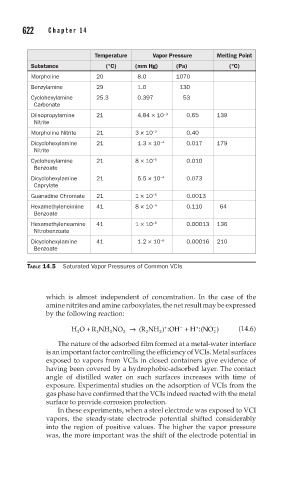
Temperature Vapor Pressure Melting Point
Substance (°C) (mm Hg) (Pa) (°C)
Morpholine 20 8.0 1070
Benzylamine 29 1.0 130
Cyclohexylamine 25.3 0.397 53
Carbonate
Diisopropylamine 21 4.84 × 10 −3 0.65 139
Nitrite
Morpholine Nitrite 21 3 × 10 −3 0.40
Dicyclohexylamine 21 1.3 × 10 −4 0.017 179
Nitrite
Cyclohexylamine 21 8 × 10 −5 0.010
Benzoate
Dicyclohexylamine 21 5.5 × 10 −4 0.073
Caprylate
Guanadine Chromate 21 1 × 10 −5 0.0013
Hexamethyleneimine 41 8 × 10 −4 0.110 64
Benzoate
Hexamethyleneamine 41 1 × 10 −6 0.00013 136
Nitrobenzoate
Dicyclohexylamine 41 1.2 × 10 −6 0.00016 210
Benzoate
TABLE 14.5 Saturated Vapor Pressures of Common VCIs
which is almost independent of concentration. In the case of the
amine nitrites and amine carboxylates, the net result may be expressed
by the following reaction:
:
H O + R NH NO → (R NH ) + : OH + H (NO ) (14.6)
−
+
−
2
2
2
2
2
2
2
The nature of the adsorbed film formed at a metal-water interface
is an important factor controlling the efficiency of VCIs. Metal surfaces
exposed to vapors from VCIs in closed containers give evidence of
having been covered by a hydrophobic-adsorbed layer. The contact
angle of distilled water on such surfaces increases with time of
exposure. Experimental studies on the adsorption of VCIs from the
gas phase have confirmed that the VCIs indeed reacted with the metal
surface to provide corrosion protection.
In these experiments, when a steel electrode was exposed to VCI
vapors, the steady-state electrode potential shifted considerably
into the region of positive values. The higher the vapor pressure
was, the more important was the shift of the electrode potential in