Page 35 - Coulson Richardson's Chemical Engineering Vol.6 Chemical Engineering Design 4th Edition
P. 35
18
CHEMICAL ENGINEERING
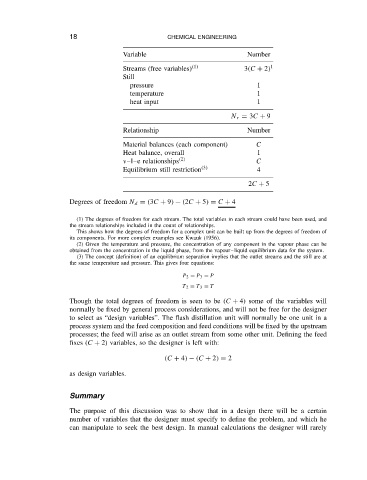
Number
Variable
Streams (free variables) 1 3 C C 2 1
Still
pressure 1
temperature 1
heat input 1
N r D 3C C 9
Relationship Number
Material balances (each component) C
Heat balance, overall 1
v l e relationships 2 C
Equilibrium still restriction 3 4
2C C 5
Degrees of freedom N d D 3C C 9 2C C 5 D C C 4
(1) The degrees of freedom for each stream. The total variables in each stream could have been used, and
the stream relationships included in the count of relationships.
This shows how the degrees of freedom for a complex unit can be built up from the degrees of freedom of
its components. For more complex examples see Kwauk (1956).
(2) Given the temperature and pressure, the concentration of any component in the vapour phase can be
obtained from the concentration in the liquid phase, from the vapour liquid equilibrium data for the system.
(3) The concept (definition) of an equilibrium separation implies that the outlet streams and the still are at
the same temperature and pressure. This gives four equations:
P 2 D P 3 D P
T 2 D T 3 D T
Though the total degrees of freedom is seen to be (C C 4) some of the variables will
normally be fixed by general process considerations, and will not be free for the designer
to select as “design variables”. The flash distillation unit will normally be one unit in a
process system and the feed composition and feed conditions will be fixed by the upstream
processes; the feed will arise as an outlet stream from some other unit. Defining the feed
fixes (C C 2) variables, so the designer is left with:
C C 4 C C 2 D 2
as design variables.
Summary
The purpose of this discussion was to show that in a design there will be a certain
number of variables that the designer must specify to define the problem, and which he
can manipulate to seek the best design. In manual calculations the designer will rarely