Page 186 - Design of Simple and Robust Process Plants
P. 186
5.5 Intensification of Process Functions 171
awayº reaction. This was the dominant reason why the process was performed
under isothermal conditions, with the reaction heat being lost into the cooling water
(see Figure 4.8 in Chapter 4). The tubular reaction was adiabatic, and the heat could
be utilized for downstream separation. This resulted not only in a considerable
reduction in reactor cost but also a reduced energy cost. The safety of the process
was improved as the hold-up on reacting components was reduced over ten-fold.
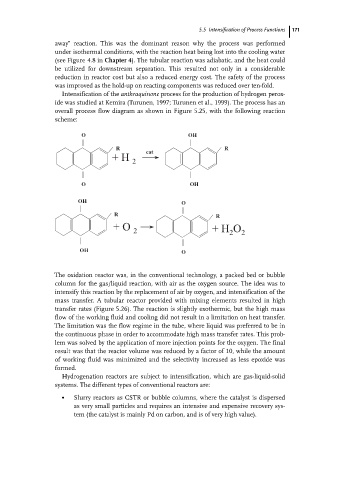
Intensification of the anthraquinone process for the production of hydrogen perox-
ide was studied at Kemira (Turunen, 1997; Turunen et al., 1999). The process has an
overall process flow diagram as shown in Figure 5.25, with the following reaction
scheme:
O OH
R cat R
+H 2
O OH
OH O
R R
+O +H O
2 2 2
OH O
The oxidation reactor was, in the conventional technology, a packed bed or bubble
column for the gas/liquid reaction, with air as the oxygen source. The idea was to
intensify this reaction by the replacement of air by oxygen, and intensification of the
mass transfer. A tubular reactor provided with mixing elements resulted in high
transfer rates (Figure 5.26). The reaction is slightly exothermic, but the high mass
flow of the working fluid and cooling did not result in a limitation on heat transfer.
The limitation was the flow regime in the tube, where liquid was preferred to be in
the continuous phase in order to accommodate high mass transfer rates. This prob-
lem was solved by the application of more injection points for the oxygen. The final
result was that the reactor volume was reduced by a factor of 10, while the amount
of working fluid was minimized and the selectivity increased as less epoxide was
formed.
Hydrogenation reactors are subject to intensification, which are gas-liquid-solid
systems. The different types of conventional reactors are:
. Slurry reactors as CSTR or bubble columns, where the catalyst is dispersed
as very small particles and requires an intensive and expensive recovery sys-
tem (the catalyst is mainly Pd on carbon, and is of very high value).