Page 138 - Distillation theory
P. 138
P1: JPJ/FFX P2: FCH/FFX QC: FCH/FFX T1: FCH
0521820928c05 CB644-Petlyuk-v1 June 11, 2004 20:15
112 Distillation Trajectories and Conditions of Mixture Separability
y f
a) x F
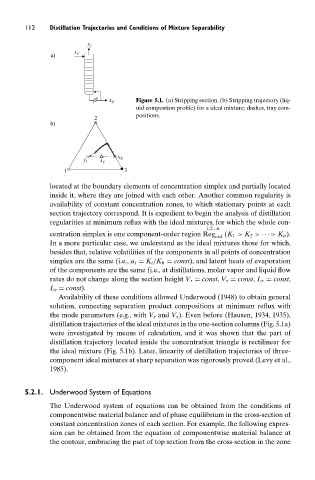
Figure 5.1. (a) Stripping section. (b) Stripping trajectory (liq-
x B
uid compostion profile) for a ideal mixture; dashes, tray com-
positions.
2
b)
x B
y f x F
1 3
located at the boundary elements of concentration simplex and partially located
inside it, where they are joined with each other. Another common regularity is
availability of constant concentration zones, to which stationary points at each
section trajectory correspond. It is expedient to begin the analysis of distillation
regularities at minimum reflux with the ideal mixtures, for which the whole con-
1,2...n
centration simplex is one component-order region Reg (K 1 > K 2 > ··· > K n ).
ord
In a more particular case, we understand as the ideal mixtures those for which,
besides that, relative volatilities of the components in all points of concentration
simplex are the same (i.e., α i = K i /K h = const), and latent heats of evaporation
of the components are the same (i.e., at distillations, molar vapor and liquid flow
rates do not change along the section height V r = const, V s = const, L r = const,
L s = const).
Availability of these conditions allowed Underwood (1948) to obtain general
solution, connecting separation product compositions at minimum reflux with
the mode parameters (e.g., with V r and V s ). Even before (Hausen, 1934, 1935),
distillation trajectories of the ideal mixtures in the one-section columns (Fig. 5.1a)
were investigated by means of calculation, and it was shown that the part of
distillation trajectory located inside the concentration triangle is rectilinear for
the ideal mixture (Fig. 5.1b). Later, linearity of distillation trajectories of three-
component ideal mixtures at sharp separation was rigorously proved (Levy et al.,
1985).
5.2.1. Underwood System of Equations
The Underwood system of equations can be obtained from the conditions of
componentwise material balance and of phase equilibrium in the cross-section of
constant concentration zones of each section. For example, the following expres-
sion can be obtained from the equation of componentwise material balance at
the contour, embracing the part of top section from the cross-section in the zone