Page 153 - Distillation theory
P. 153
P1: JPJ/FFX P2: FCH/FFX QC: FCH/FFX T1: FCH
0521820928c05 CB644-Petlyuk-v1 June 11, 2004 20:15
5.4 Evolution of Section Trajectory Bundles for Three-Component Mixture 127
2 2
a) b)
1 3 1 3
+ +
N S N
x D 2 x D S 3 2
c) d)
x x F
F S 2
( V)L = 1
3
S
x B x B
(L V)
S 1
2
(L V)
x D x 1
D
1 + 3 1 + + 3
− N − 1 N 2 N +
N N N
3
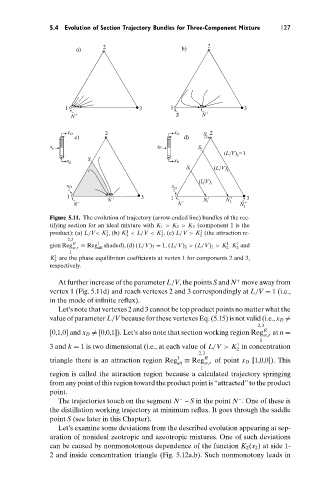
Figure 5.11. The evolution of trajectory (arrow-ended line) bundles of the rec-
tifying section for an ideal mixture with K 1 > K 2 > K 3 (component 1 is the
1
1
1
1
product): (a) L/V< K , (b) K < L/V < K , (c) L/V > K (the attraction re-
3
2
2
3
2,3
1
1
R
gion Reg w,r ≡ Reg 1 att shaded), (d) (L/V) 3 = 1, (L/V) 2 > (L/V) 1 > K . K and
2
2
1
1
K are the phase equilibrium coefficients at vertex 1 for components 2 and 3,
3
respectively.
At further increase of the parameter L/V, the points S and N move away from
+
vertex 1 (Fig. 5.11d) and reach vertexes 2 and 3 correspondingly at L/V = 1 (i.e.,
in the mode of infinite reflux).
Let’s note that vertexes 2 and 3 cannot be top product points no matter what the
value of parameter L/V because for these vertexes Eq. (5.15) is not valid (i.e., x D
=
2,3
[0,1,0] and x D
= [0,0,1]). Let’s also note that section working region Reg R at n =
w,r
1
1
3 and k = 1 is two dimensional (i.e., at each value of L/V > K in concentration
2
2,3
triangle there is an attraction region Reg 1 att ≡ Reg R of point x D [1,0,0]). This
w,r
1
region is called the attraction region because a calculated trajectory springing
from any point of this region toward the product point is “attracted” to the product
point.
−
The trajectories touch on the segment N – S in the point N . One of these is
−
the distillation working trajectory at minimum reflux. It goes through the saddle
point S (see later in this Chapter).
Let’s examine some deviations from the described evolution appearing at sep-
aration of nonideal zeotropic and azeotropic mixtures. One of such deviations
can be caused by nonmonotonous dependence of the function K 2 (x 2 ) at side 1-
2 and inside concentration triangle (Fig. 5.12a,b). Such nonmonotony leads in