Page 148 - Distillation theory
P. 148
P1: JPJ/FFX P2: FCH/FFX QC: FCH/FFX T1: FCH
0521820928c05 CB644-Petlyuk-v1 June 11, 2004 20:15
122 Distillation Trajectories and Conditions of Mixture Separability
k+1
x
lg k
x
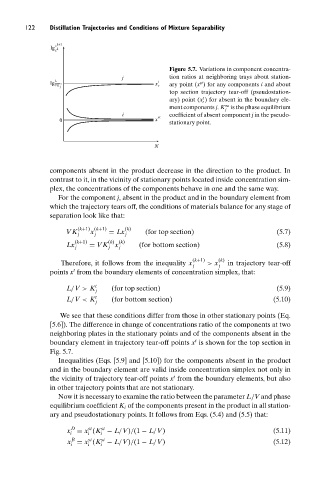
Figure 5.7. Variations in component concentra-
j tion ratios at neighboring trays about station-
L t
st
lg VK ∞ x ary point (x ) for any components i and about
j r
top section trajectory tear-off (pseudostation-
t
ary) point (x ) for absent in the boundary ele-
r
∞
ment components j. K is the phase equilibrium
j
i coefficient of absent component j in the pseudo-
0 x st stationary point.
N
components absent in the product decrease in the direction to the product. In
contrast to it, in the vicinity of stationary points located inside concentration sim-
plex, the concentrations of the components behave in one and the same way.
For the component j, absent in the product and in the boundary element from
which the trajectory tears off, the conditions of materials balance for any stage of
separation look like that:
(k+1) (k+1) (k)
VK x = Lx (for top section) (5.7)
j j j
(k+1) (k) (k)
Lx j = VK j x j (for bottom section) (5.8)
(k+1) (k)
Therefore, it follows from the inequality x > x in trajectory tear-off
j j
t
points x from the boundary elements of concentration simplex, that:
L/V > K t (for top section) (5.9)
j
L/V < K t (for bottom section) (5.10)
j
We see that these conditions differ from those in other stationary points (Eq.
[5.6]). The difference in change of concentrations ratio of the components at two
neighboring plates in the stationary points and of the components absent in the
t
boundary element in trajectory tear-off points x is shown for the top section in
Fig. 5.7.
Inequalities (Eqs. [5.9] and [5.10]) for the components absent in the product
and in the boundary element are valid inside concentration simplex not only in
t
the vicinity of trajectory tear-off points x from the boundary elements, but also
in other trajectory points that are not stationary.
Now it is necessary to examine the ratio between the parameter L/V and phase
equilibrium coefficient K i of the components present in the product in all station-
ary and pseudostationary points. It follows from Eqs. (5.4) and (5.5) that:
D
st
st
x = x (K − L/V)/(1 − L/V) (5.11)
i i i
st
B
st
x = x (K − L/V)/(1 − L/V) (5.12)
i i i