Page 52 - Distillation theory
P. 52
P1: FCH/FFX P2: FCH/FFX QC: VINOD/IYP T1: FCH
0521820928c02 CB644-Petlyuk-v1 June 11, 2004 17:58
26 Basic Concepts of Distillation
2 2
a) b)
x
x B(1)
D(2) x
x D(4) B(3)
x x B
x D(3) F x B(4) x F
x D
1 3 1 3
x D(1) x B(2)
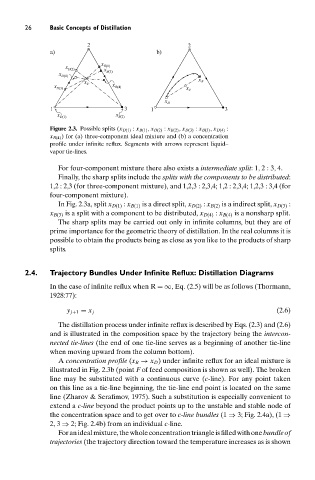
Figure 2.3. Possible splits (x D(1) : x B(1) , x D(2) : x B(2) , x D(3) : x B(3) , x D(4) :
x B(4) ) for (a) three-component ideal mixture and (b) a concentration
profile under infinite reflux. Segments with arrows represent liquid–
vapor tie-lines.
For four-component mixture there also exists a intermediate split:1, 2:3, 4.
Finally, the sharp splits include the splits with the components to be distributed:
1,2 : 2,3 (for three-component mixture), and 1,2,3 : 2,3,4; 1,2 : 2,3,4; 1,2,3 : 3,4 (for
four-component mixture).
In Fig. 2.3a, split x D(1) : x B(1) is a direct split, x D(2) : x B(2) is a indirect split, x D(3) :
x B(3) is a split with a component to be distributed, x D(4) : x B(4) is a nonsharp split.
The sharp splits may be carried out only in infinite columns, but they are of
prime importance for the geometric theory of distillation. In the real columns it is
possible to obtain the products being as close as you like to the products of sharp
splits.
2.4. Trajectory Bundles Under Infinite Reflux: Distillation Diagrams
In the case of infinite reflux when R =∞, Eq. (2.5) will be as follows (Thormann,
1928:77):
y j+1 = x j (2.6)
The distillation process under infinite reflux is described by Eqs. (2.3) and (2.6)
and is illustrated in the composition space by the trajectory being the intercon-
nected tie-lines (the end of one tie-line serves as a beginning of another tie-line
when moving upward from the column bottom).
A concentration profile (x B → x D ) under infinite reflux for an ideal mixture is
illustrated in Fig. 2.3b (point F of feed composition is shown as well). The broken
line may be substituted with a continuous curve (c-line). For any point taken
on this line as a tie-line beginning, the tie-line end point is located on the same
line (Zharov & Serafimov, 1975). Such a substitution is especially convenient to
extend a c-line beyond the product points up to the unstable and stable node of
the concentration space and to get over to c-line bundles (1 ⇒ 3; Fig. 2.4a), (1 ⇒
2, 3 ⇒ 2; Fig. 2.4b) from an individual c-line.
Foranidealmixture,thewholeconcentrationtriangleisfilledwithonebundleof
trajectories (the trajectory direction toward the temperature increases as is shown