Page 167 - Drilling Technology in Nontechnical Language
P. 167
158 Drilling Technology in Nontechnical Language Second Edition
Dispersed muds
In the first part of chapter 1, clay mineral types and clay hydration
were discussed. Some clays react strongly with water (they hydrate) and
will expand in the presence of water. Water that is allowed to enter the
crystal structure can cause the crystal lattice to expand because of changes
in electrostatic forces. This expansion is described as dispersion. Water
molecules are polar; that is, the water molecule, while being electrically
neutral overall, has positively and negatively charged areas in different
parts of the molecule. The polar nature of water can be increased by the
addition of alkalis such as sodium or potassium hydroxide. The more polar
the water, the more that reactive clays will disperse.
The potential reactivity of a clay formation will depend on the types
of clays present and the physical environment. Some clays are more likely
to hydrate and expand than others. One highly reactive clay mineral in the
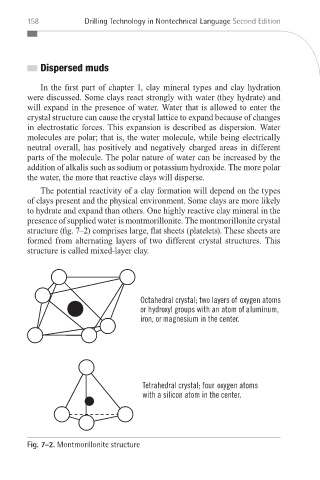
presence of supplied water is montmorillonite. The montmorillonite crystal
structure (fig. 7–2) comprises large, flat sheets (platelets). These sheets are
formed from alternating layers of two different crystal structures. This
structure is called mixed-layer clay.
Fig. 7–2. Montmorillonite structure
_Devereux_Book.indb 158 1/16/12 2:09 PM