Page 300 - Electrical Properties of Materials
P. 300
282 Magnetic materials
In Weiss’s classical picture the magnetic moments are lined up by a long-
range internal field. In the quantum picture they are lined up owing to
∗
∗ F. Keffer, Magnetic properties of ma- nearest-neighbour interaction. ‘One is reminded,’ writes Keffer , ‘of the situ-
terials, Scientific American,Septem- ation when, as the quiet of evening descends, suddenly all the dogs in a town
ber 1967.
get to barking together, although each dog responds only to the neighbouring
dogs.’
11.7.6 Ferrimagnetism
This type of magnetism occurs in compounds only, where the exchange interac-
tion causes the electrons of each set of atoms to line up parallel, but the two sets
are antiparallel to each other. If the magnetic moments are unequal, then we get
the situation shown in Fig. 11.19(c), where the resultant magnetic moment may
be quite large. For most practical purposes ferrimagnetic materials behave like
ferromagnetics but have a somewhat lower saturation magnetization.
11.7.7 Garnets
This is the name for a class of compounds crystallizing in a certain crystal
structure. As far as magnetic properties are concerned, their most interest-
ing representative is yttrium-iron garnet (Y 3 Fe 5 O 12 ), which happens to be
ferromagnetic for a rather curious reason. The spin of the yttrium atoms is
opposite to the spin of the iron atoms, so the magnetic moments would line up
alternately—if the orbital magnetic moments were small. But for yttrium the
orbital magnetic moment is large, larger actually than the spin, and is in the
opposite direction. Hence, the total magnetic moment of the yttrium atom is in
the same direction as that of iron, making the compound ferromagnetic.
11.7.8 Helimagnetism
You may wonder why the magnetic moments of neighbouring atoms in an
ordered structure are either parallel or antiparallel. One would expect quantum
mechanics to produce a larger variety. In actual fact, there are some materials
in which the spins in a given atomic layer are all in the same direction, but
◦
the spins of adjacent layers lie at an angle (e.g. 129 in MnO 2 below a cer-
tain temperature), producing a kind of helix. For the moment this is a scientific
curiosity with no practical application.
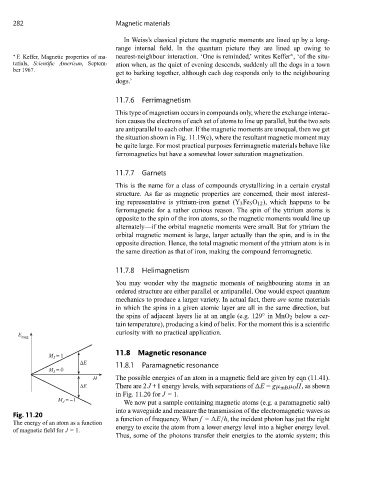
E
mag
11.8 Magnetic resonance
M = 1
J
ΔE 11.8.1 Paramagnetic resonance
M = 0
J
H The possible energies of an atom in a magnetic field are given by eqn (11.41).
ΔE There are 2 J +1 energy levels, with separations of E = gμ mB μ 0 H,asshown
in Fig. 11.20 for J =1.
M = –1 We now put a sample containing magnetic atoms (e.g. a paramagnetic salt)
J
into a waveguide and measure the transmission of the electromagnetic waves as
Fig. 11.20
a function of frequency. When f = E/h, the incident photon has just the right
Theenergyofanatomasafunction
energy to excite the atom from a lower energy level into a higher energy level.
of magnetic field for J =1.
Thus, some of the photons transfer their energies to the atomic system; this