Page 60 - Electrical Properties of Materials
P. 60
The electron meeting a potential barrier 43
Now we shall ask the question, depending on the relative magnitudes of E
and V 2 , what is the probability that the electron can be found in regions 1 and
2, respectively.
It is actually easier to speak about this problem in wave language because
then the form of the solution is automatically suggested. Whenever a wave is
incident on some sort of discontinuity, there is a reflected wave, and there is a
transmitted wave. Since no wave is incident from region 2, we can immediately
decide that D must be zero.
In order to determine the remaining constants, we have to match the two
solutions at z = 0, requiring that both ψ and ∂ψ/∂z should be continuous. From
eqns (3.21) and (3.24), the above conditions lead to the algebraic equations,
A + B = C (3.26)
and
ik 1 (A – B)=ik 2 C, (3.27)
whence
B k 1 – k 2 C 2k 1
= , = . (3.28)
A k 1 + k 2 A k 1 + k 2
2
Let us distinguish now two cases: (i) E > V 2 . In this case k > 0, k 2 is
2
real, which means an oscillatory solution in region 2. The values of k 2 and k 1
are, however, different. Thus, B/A is finite, that is, there is a finite amount of ∗ The exponentially increasing solution
reflection. In contrast to the classical solution, there is some probability that cannot be present for physical reasons.
the electron is turned back by the potential discontinuity. (ii) E < V 2 .Inthis
2
case k < 0, k 2 is imaginary; that is, the solution declines exponentially in
2 1 2 3
∗
region 2. Since |C/A| > 0, there is a finite, though declining, probability
V
of finding the electron at z > 0. Classically, an electron has no chance of
Electron
getting inside region 2. Under the laws of quantum mechanics the electron
may penetrate the potential barrier. V 2 V = 0
3
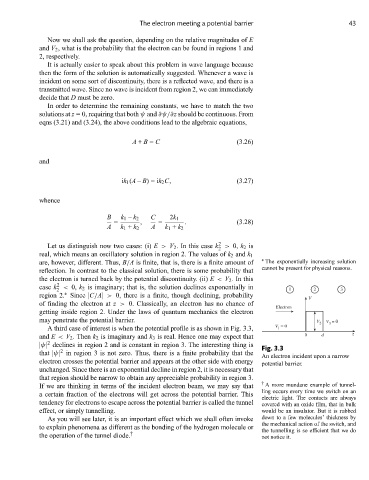
A third case of interest is when the potential profile is as shown in Fig. 3.3, V = 0
1
and E < V 2 . Then k 2 is imaginary and k 3 is real. Hence one may expect that 0 d z
2
|ψ| declines in region 2 and is constant in region 3. The interesting thing is Fig. 3.3
2
that |ψ| in region 3 is not zero. Thus, there is a finite probability that the
An electron incident upon a narrow
electron crosses the potential barrier and appears at the other side with energy potential barrier.
unchanged. Since there is an exponential decline in region 2, it is necessary that
that region should be narrow to obtain any appreciable probability in region 3.
†
If we are thinking in terms of the incident electron beam, we may say that A more mundane example of tunnel-
ling occurs every time we switch on an
a certain fraction of the electrons will get across the potential barrier. This
electric light. The contacts are always
tendency for electrons to escape across the potential barrier is called the tunnel covered with an oxide film, that in bulk
effect, or simply tunnelling. would be an insulator. But it is rubbed
As you will see later, it is an important effect which we shall often invoke down to a few molecules’ thickness by
the mechanical action of the switch, and
to explain phenomena as different as the bonding of the hydrogen molecule or the tunnelling is so efficient that we do
the operation of the tunnel diode. † not notice it.