Page 82 - Electrical Properties of Materials
P. 82
64 The hydrogen atom and the periodic table
1 2
IA IIA H He IIIB IVB VB VIB VIIB
3 4 5 6 7 8 9 10
Li Be B C N O F Ne
11 12 13 14 15 16 17 18
Na Mg IIIA IVA VA VIA VIIA VIII IB IIB Al Si P S Cl Ar
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
87 88 89
Fr Ra Ti
58 59 60 61 62 63 64 65 66 67 68 69 70 71
102 } IIIA
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
90 91 92 93 94 95 96 97 98 99 100 101 103
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No
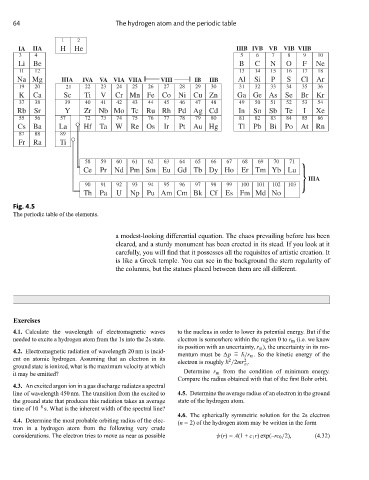
Fig. 4.5
The periodic table of the elements.
a modest-looking differential equation. The chaos prevailing before has been
cleared, and a sturdy monument has been erected in its stead. If you look at it
carefully, you will find that it possesses all the requisites of artistic creation. It
is like a Greek temple. You can see in the background the stern regularity of
the columns, but the statues placed between them are all different.
Exercises
4.1. Calculate the wavelength of electromagnetic waves to the nucleus in order to lower its potential energy. But if the
needed to excite a hydrogen atom from the 1s into the 2s state. electron is somewhere within the region 0 to r m (i.e. we know
its position with an uncertainty, r m ), the uncertainty in its mo-
4.2. Electromagnetic radiation of wavelength 20 nm is incid- ~
mentum must be p = /r m . So the kinetic energy of the
ent on atomic hydrogen. Assuming that an electron in its 2 2
electron is roughly /2mr .
ground state is ionized, what is the maximum velocity at which m
Determine r m from the condition of minimum energy.
it may be emitted?
Compare the radius obtained with that of the first Bohr orbit.
4.3. An excited argon ion in a gas discharge radiates a spectral
line of wavelength 450 nm. The transition from the excited to 4.5. Determine the average radius of an electron in the ground
the ground state that produces this radiation takes an average state of the hydrogen atom.
–8
time of 10 s. What is the inherent width of the spectral line?
4.6. The spherically symmetric solution for the 2s electron
4.4. Determine the most probable orbiting radius of the elec- (n = 2) of the hydrogen atom may be written in the form
tron in a hydrogen atom from the following very crude
considerations. The electron tries to move as near as possible ψ(r)= A(1 + c 1 r)exp(–rc 0 /2), (4.32)