Page 139 - Bruno Linder Elementary Physical Chemistry
P. 139
August 19, 2010 10:40 9in x 6in b985-ch12 Elementary Physical Chemistry
124 Elementary Physical Chemistry
(c) Surface Tension. This is the energy needed to increase the surface area
of liquids. To increase the surface, it is necessary to pull the molecules
apart against the attractive forces. Thus, the surface tension increases
with strength of IF.
(d) Viscosity. This is a measure of the resistance to flow. The stronger the
inter molecular forces, the higher the viscosity.
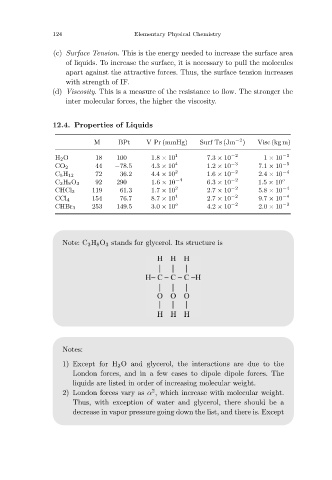
12.4. Properties of Liquids
M BPt V Pr (mmHg) Surf Ts (Jm −2 ) Visc (kg m)
H 2O 18 100 1.8 × 10 1 7.3 × 10 −2 1 × 10 −3
CO 2 44 −78.5 4.3 × 10 4 1.2 × 10 −3 7.1 × 10 −5
C 5H 12 72 36.2 4.4 × 10 2 1.6 × 10 −2 2.4 × 10 −4
C 3H 8O 3 92 290 1.6 × 10 −4 6.3 × 10 −2 1.5 × 10 o
CHCl 3 119 61.3 1.7 × 10 2 2.7 × 10 −2 5.8 × 10 −4
CCl 4 154 76.7 8.7 × 10 1 2.7 × 10 −2 9.7 × 10 −4
CHBr 3 253 149.5 3.0 × 10 o 4.2 × 10 −2 2.0 × 10 −3
Note: C 3 H 8 O 3 stands for glycerol. Its structure is
Notes:
1) Except for H 2 O and glycerol, the interactions are due to the
London forces, and in a few cases to dipole–dipole forces. The
liquids are listed in order of increasing molecular weight.
2
2) London forces vary as α , which increase with molecular weight.
Thus, with exception of water and glycerol, there should be a
decrease in vapor pressure going down the list, and there is. Except