Page 134 - Bruno Linder Elementary Physical Chemistry
P. 134
August 18, 2010 11:37 9in x 6in b985-ch11 Elementary Physical Chemistry
Elements of Molecular Spectroscopy 119
1
35
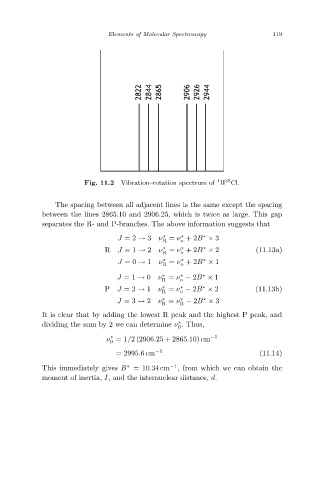
Vibration–rotation spectrum of H Cl.
Fig. 11.2
The spacing between all adjacent lines is the same except the spacing
between the lines 2865.10 and 2906.25, which is twice as large. This gap
separates the R- and P-branches. The above information suggests that
∗
∗
J =2 → 3 ν = ν +2B × 3
∗
R o
∗
R J =1 → 2 ν = ν +2B × 2 (11.13a)
∗
∗
R o
∗
∗
J =0 → 1 ν = ν +2B × 1
∗
R o
∗
∗
J =1 → 0 ν = ν − 2B × 1
∗
R
o
∗
∗
∗
P J =2 → 1 ν = ν − 2B × 2 (11.13b)
o
R
∗
∗
∗
J =3 → 2 ν = ν − 2B × 3
R
R
It is clear that by adding the lowest R peak and the highest P peak, and
dividing the sum by 2 we can determine ν .Thus,
∗
0
∗
ν =1/2 (2906.25 + 2865.10) cm −1
0
= 2995.6cm −1 (11.14)
This immediately gives B =10.34cm −1 , from which we can obtain the
∗
moment of inertia, I, and the internuclear distance, d.