Page 123 - Elements of Chemical Reaction Engineering 3rd Edition
P. 123
Sec. 3.3 Stoichiometric Table 95
-7
Gas-pilase
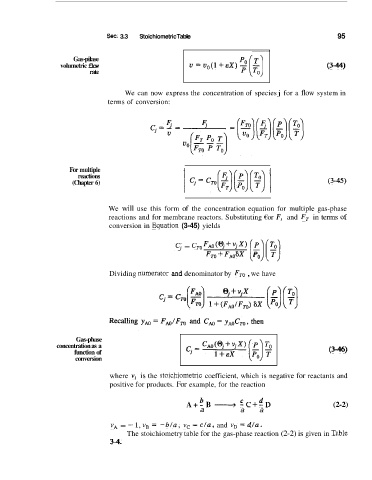
volumetric flow U=u,(l+EX)- (3-44)
rate
We can now express the concentration of species j for a flow system in
terms of conversion:
For multiple
reactions
(Chapter 6) (3-45)
We will use this form of the concentration equation for multiple gas-phase
reactions and for membrane reactors. Substituting €or F, and FT in terms of
conversion in Eqluation (3-45) yields
'Cj = c, FAO(q+vjx) [E)[:)
FTO+FAOsx
Dividing numera.tor and denominator by FTO , we have
Gas-phase
concentration as a (3m.46)
function of
conversion
where vi is the !stoichiometric coefficient, which is negative for reactants and
positive for products. For example, for the reaction
b c d
A+ - B + -C+--D (2-2)
a a a
vA = -- 1, vB = -b/a, vc = c/a, and vD = dla.
The stoichiometry table for the gas-phase reaction (2-2) is given in T.able
3-4.