Page 164 - Elements of Chemical Reaction Engineering 3rd Edition
P. 164
136 Isothermal Reactor Design Chap. 4
1 Rearranging and taking the logarithm of both sides yields
(E4- 1.73
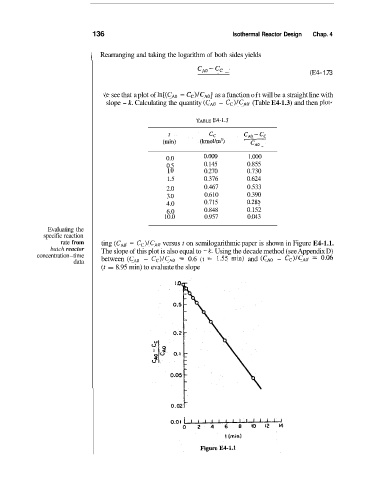
Ve see that a plot of ln[(CAo - cC)/cAo] as a function oft will be a straight line with
slope - k. Calculating the quantity (CAo - Cc)/CAo (Table E4-1.3) and then plot-
'rABLE FA-1.3
0.0 O.OO0 1.000
0.5 0.145 0.855
1 .o 0.270 0.730
1.5 0.376 0.624
2.0 0.467 0.533
3 .O 0.610 0.390
4.0 0.715 0.285
6.0 0.848 0.152
10.0 0.957 0.043
Evaluating the
specific reaction
rate from ting (CAo - Cc)/CAo versus t on semilogarithmic paper is shown in Figure E4-1.1.
batch reactor The slope of this plot is also equal to -k. Using the decade method (see Appendix D)
concentration-time between (CAo - Cc)/CAo = 0.6 (t ;= 1.55 min) and (CA0 - CC)/~AO 0.06
=
data
(t = 8.95 min) to evaluate the slope
0.02 1