Page 166 - Elements of Chemical Reaction Engineering 3rd Edition
P. 166
1 38 Isothermal Rqactor Design Chap. 4
For a first-order reactioq the product zk is often referred to as the reaction
Da = - Damkohler number.
FAO
The Damkohler is a dimensionless number that can give us a quick esti-
mate of the degree of conversion that can be achieved in continuous-flow reac-
tors. The Damkohler number is the ratio of the rate of reaction of A to the rate
of convective transport of A at the entrance to the reactor. For first- and sec-
ond-order irreversible reactions the Damkijhler numbers are
and
respectively. It is important to know what values of the Dardcohler number,
Da, give high and low conversion in continuous-flow reactors. A value of
Da = 0.1 or less will usually give less than 10% conversion and a value of
0.1 <Da< 10 Da = 10.0 or greater will usually give greater than 90% conversion.
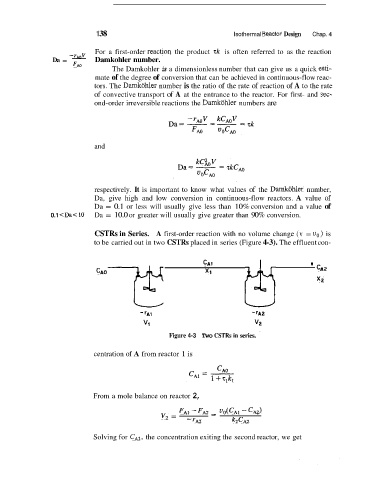
CSTRs in Series. A first-order reaction with no volume change (v = u,) is
to be carried out in two CSTRs placed in series (Figure 4-3). The effluent con-
CAI
CAO XI ' cA2
X2
Figure 4-3 Two CSTRs in series.
centration of A from reactor 1 is
From a mole balance on reactor 2,
- FA2 - - vO(cAl -
vz =
-'A2 k2CA2
Solving for CA2, the concentration exiting the second reactor, we get