Page 170 - Elements of Chemical Reaction Engineering 3rd Edition
P. 170
T ":"I Isothermal Reacto- Design Chap. 4
142
I .o
I
I
1 I
0.4 I
I
1 I
I I
I I
I I
I I
I I
I I
1 I
I I
I 1 I I I IIIP I 1 I IIJ
0.1 0.2 0.4 0.6 1.0 2 4 6 IO 20 40 60
rkCAO
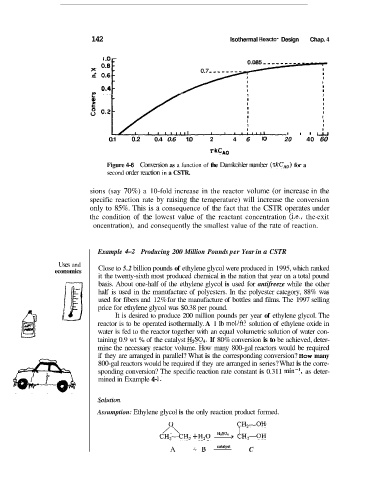
Figure 4-6 Conversion as a function of the Damkohler number (.rkCA0) for a
second order reaction in a CSTR.
sions (say 70%) a 10-fold increase in the reactor volume (or increase in the
specific reaction rate by raising the temperature) will increase the conversion
only to 85%. This is a consequence of the fact that the CSTR operates under
the condition of the lowest value of the reactant concentration (i.e., the-exit
oncentration), and consequently the smallest value of the rate of reaction.
Example 4-2 Producing 200 Million Pounds per Year in a CSTR
Uses and
economics Close to 5.2 billion pounds of ethylene glycol were produced in 1995, which ranked
it the twenty-sixth most produced chemical in the nation that year on a total pound
basis. About one-half of the ethylene glycol is used for antifreeze while the other
half is used in the manufacture of polyesters. In the polyester category, 88% was
used for fibers and 12% for the manufacture of bottles and films. The 1997 selling
price for ethylene glycol was $0.38 per pound.
It is desired to produce 200 million pounds per year of ethylene glycol. The
reactor is to be operated isothermally. A 1 lb mol/ft3 solution of ethylene oxide in
water is fed to the reactor together with an equal volumetric solution of water con-
taining 0.9 wt % of the catalyst &SO4. If 80% conversion is to be achieved, deter-
mine the necessary reactor volume. How many 800-gal reactors would be required
if they are arranged in parallel? What is the corresponding conversion? How many
800-gal reactors would be required if they are arranged in series? What is the corre-
sponding conversion? The specific reaction rate constant is 0.311 min-', as deter-
I I .b- 1
mined in Example 4- 1,
Suludiun
Assumption: Ethylene glycol is the only reaction product formed.
CH,-OH
/"\ I
CH,-CH, + H,O H2so' > CH,-OH
A +B catalyst C