Page 173 - Elements of Chemical Reaction Engineering 3rd Edition
P. 173
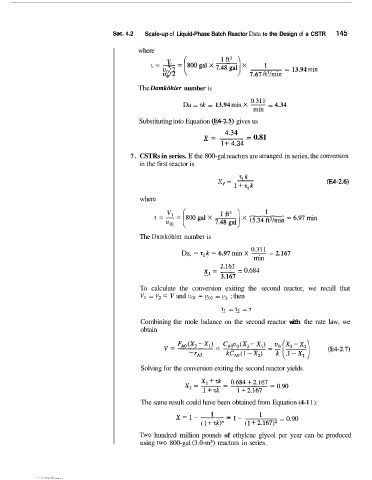
Sec. 4..2 Scale-up of Liquid-Phase Batch Reactor Data to the Design of a CSTR 145
where
1 ft3
z== -=~galx~al)x = 13.94 inin
V
v0/2 7.67 ft3/min
The Damkiihler number is
Da = 7k = 13.94 min X 0.311 = 4.34
min
Substituting into Equation (E%-2.5) gives us
x= -_ 4'34 - 0.81
1 i- 4.34
7. CSTRs in series. If the 800-gal reactors are arrmged in series, the cwmion
in the first reactor is
9 k
x, = - W-2.6)
1+T,k
where
The Dmkohler number is
Da, =: ~,k 6.97 min X os
=
rnin = 2.167
x,= 2'167 - 0.684
--
3.167
To calculate the conversion exiting the second reactor, we recall that
V1 = V2 = V and uol = vO2 = vo ; then
= 7.2 = 7
Combining the mole balance on the second reactor with the rate law, we
obtain
Solving for the conversion exiting the second reactor yields
The same result could have been obtained from Equation (4-1 1):
x=1--- 1 -1- = 0.90
(1 47k)n (1 +2.167)2
Two hundred million pounds of ethylene glycol per year can be produced
using two 800-gal (3.0-m3) reactors in series.