Page 177 - Elements of Chemical Reaction Engineering 3rd Edition
P. 177
Sec. 4.3 Tubular Reactors 1 49
decreases with increasing conversion, and the reactant spends more time in the
reactor than reactants that produce no net change in the total number of moles
(e.g., .A + B and E = 0). Similarly, reactants that produce an increase in the
total rmmber of moles upon reaction (e.g., E = 2) will spend less time iin the
reactor than reactants of reactions for which E is zero or negative.
Example 4-3 Neglecting Volume Change with Reaction
The gas-phase cracking reaction
A __$ 2B-I-C
is to be carried out in a tubular reactor. The reaction is second-order and the param-
eter values are the same as those used to construct Figure 4-7. If 60% conversilon is
desired, what error will result if volume change is neglected ( E = 0 ) in sizing the
reactor?
Solution
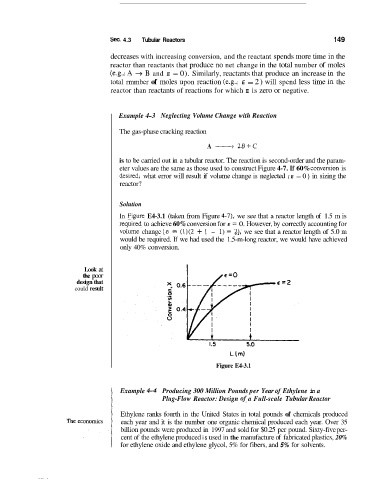
In Fiigure E4-3.1 (taken from Figure 4-3, we see that a reactor length of 1.5 m is
requiired to achieve 60% conversion for E = 0. However, by correctly accounting for
voluine change [E = (1)(2 + 1 - 1) = 23, we see that a reactor length of 5.0 m
would be required. If we had used the 1.5-m-long reactor, we would have achieved
only 40% conversion.
Look at
the poor
design that X
could result
L(m)
Figure E4-3.1
Example 4-4 Producing 300 Million Pounds per Year of Ethylene in a
Plug-Flow Reactor: Design of a Full-scale Tubular Reactor
Ethylene ranks fourth in the United States in total pounds of chemicals produced
each year and it is the number one organic chemical produced each year. Over 35
billion pounds were produced in 1997 and sold for $0.25 per pound. Sixty-five per-
cent of the ethylene produced is used in the manufacture of fabricated plastics, 20%
for ethylene oxide and ethylene glycol, 5% for fibers, and 5% for solvents.
.