Page 242 - Elements of Chemical Reaction Engineering 3rd Edition
P. 242
214 Isothermal Reactor Design Chap. 4
rently 14.1 % conversion is realized. The entering pressure is 20 atm and the
pressure at the exit of the reactor is 9.0 atmospheres. It is believed that this
reaction is internal diffusion limited. We know from Chapter 12 of Elements
of CRE (e.g. P4-23c or Eqn. 12-35, page 751) that for internal diffusion lim-
itations the rate of reaction varies inversely with the catalyst particle size.
Consequently one of the engineers suggests that the catalyst be ground up
into a smaller size. She also notes the smallest size to which the catalyst may
be ground is 0.01 cm. and that there are 3 other pipe sizes available into
which the catalyst could be packed. These non-corrosive heat-resistant pipes,
which can be cut to any length, are 2 cm, 3 cm, and 6 cm in diameter.
(a) What conversion could be achieved in a CSTR with the same catalyst
weight and no AP? (Ans.: X = 0.18 .)
(b) Calculate the maximum value of the pressure drop parameter, a, that you
can have and still maintain an exit pressure of 1 atm. (Ans.:
a = 9.975 X kg-I.)
(c) Should you change the catalyst size and pipe diameter in which 1000 kg
of the catalyst is packed while maintaining the catalyst weight?
(d) Next consider how 01 would change if you changed both pipe size and
particle size. Can you change pipe size and particle size at the same time
such that a remains constant at the value calculated in part (b)?
(e) For the conditions of part (a) [i.e., maintain a constant at the value in
part (a)], pick a pipe size and calculate a new particle size. (Ans.:
D, = 0.044 cm.) Assume turbulent flow.
(f) Calculate a new specific reaction rate ratio assuming (Le., recall the
effectiveness factor from Chapter 12) that
(g) Using the new values qf k and a, calculate the conversion for a PBR for
the new particle size for an exit pressure of 1 atm. (Ans.: X = 0.78 .)
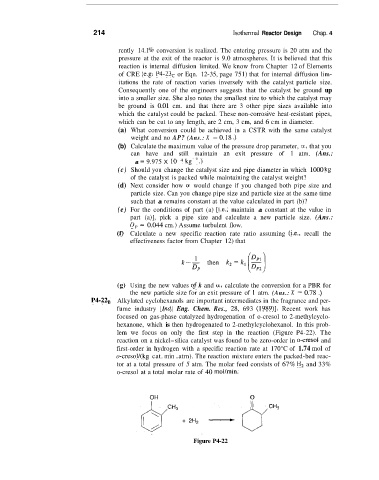
P4-22B Alkylated cyclohexanols are important intermediates in the fragrance and per-
fume industry [Ind. Eng. Chem. Res., 28, 693 (1989)l. Recent work has
focused on gas-phase catalyzed hydrogenation of o-cresol to 2-methylcyclo-
hexanone, which is then hydrogenated to 2-methylcyclohexanol. In this prob-
lem we focus on only the first step in the reaction (Figure P4-22). The
reaction on a nickel-silica catalyst was found to be zero-order in 0-cresol and
first-order in hydrogen with a specific reaction rate at 170°C of 1.74 mol of
o-cresoll(kg cat. min . atrn). The reaction mixture enters the packed-bed reac-
tor at a total pressure of 5 atm. The molar feed consists of 67% H, and 33%
o-cresol at a total molar rate of 40 mol/min.
OH 0
Figure P4-22