Page 47 - Elements of Chemical Reaction Engineering 3rd Edition
P. 47
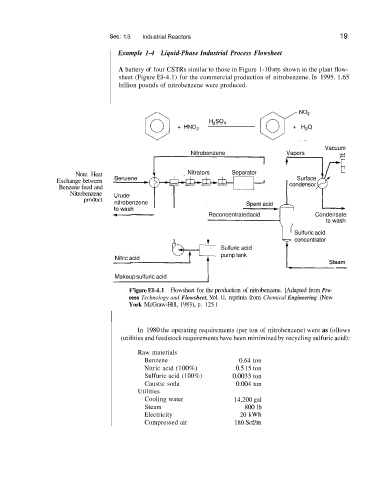
Sec. 1 .!5 Industrial Reactors 19
Example 1-4 Liquid-Phase Industrial Process Flowsheet
A battery of four CSTRs similar to those in Figure 1-10 ace shown in the plant flow-
sheet (Figure El-4.1) for the commercial production of nitrobenzene. In 1995. 1.65
billion pounds of nitrobenzene were produced.
Vacuum
Nitrobenzene Vapors
Note. Heat Nitrators Separator
Exchange between Benzene Surface
Benzene feed and
Nitrobenzene Crude
product nitrobenzene
c- Reconcentrated acid Condensate
to wash
Sulfuric acid
concentrator
Sulfuric acid
- pump tank Steam
Nitric acid
-
- I
Makeup sulfuric acid
F’igure El-4.1 Flowsheet for the production of nitrobenzene. [Adapted from Pro-
cess Technology and Flowsheet, Vol. 11, reprints from Chemical Engineering (New
York McGraw-Hill, 1983), p. 125.1
In 1980 the operating requirements (per ton of nitrobenzene) were as follows
(utilities and feedstock requirements have been minimized by recycling sulfuric acid):
Raw materials
Benzene 0.64 ton
Nitric acid (100%) 0.5 15 ton
Sulfuric acid (100%) 0.0033 ton
Caustic soda 0.004 ton
Utilities
Cooling water 14,200 gal
Steam 800 lb
Electricity 20 kWh
Compressed air 180 Scf/m